Biopharma
FlowCam for Cell Therapy Quality Control
Novel cell therapies show great promise as treatments for a variety of complex diseases including cancers and neurodegenerative disorders. Flow imaging microscopy supports drug product analysis in order to advance the development of these therapies.
For quality control of CAR-T cells and other types of cell-based medicinal products, FlowCam monitors concentrations of viable and nonviable cells, cell clusters, and particulate impurities.
For the development of organoids for disease modeling and novel therapeutic drug screening, FlowCam offers image-based surveillance to monitor culture growth characteristics in real-time.

Use FlowCam to:
- Monitor for hard-to-remove, cell-derived impurities like nonviable cells and cell debris
- Analyze aggregation of cells into large cell clusters and organoids
- Identify non-cellular contaminants including residual DynabeadsTM and protein aggregates
- Characterize particles ranging from 2 μm to 1 mm with industry-leading image quality
- Obtain particle type and morphology information to complement orthogonal sizing and counting techniques
Helpful Resources
“less laborious, inexpensive techniques that allow for rapid and reliable counting of CBMPs [cell-based medicinal products] would be beneficial for improving the quality and success of CBMPs in clinical practice. Emerging flow imaging microscopy (FIM) techniques may fulfill these needs."

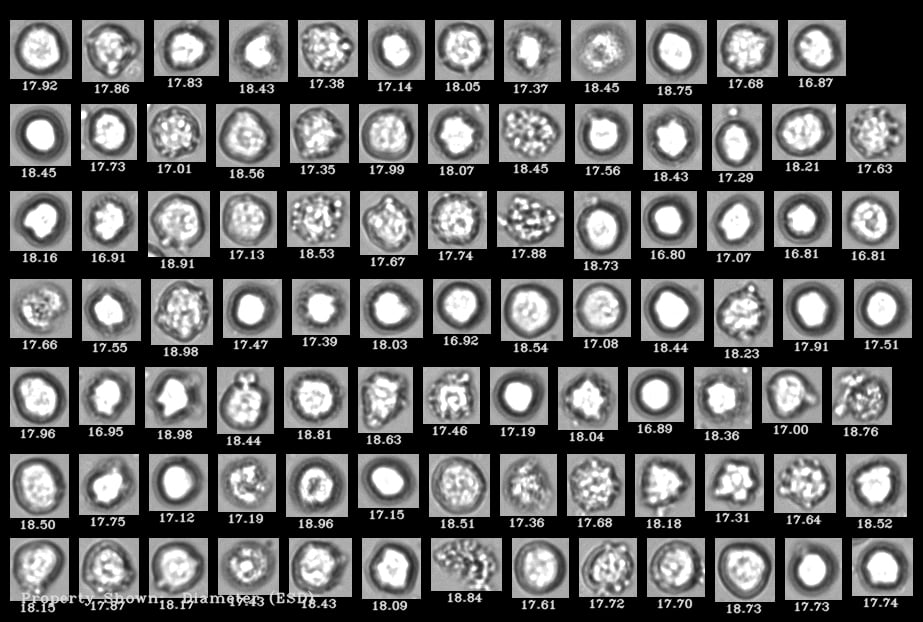
A FlowCam collage of human cells where living cells are distinguishable from dead cells

Sample FlowCam images of particles typically found in CAR-T therapies, including T-cells, cell debris, and protein aggregates from cell culture media
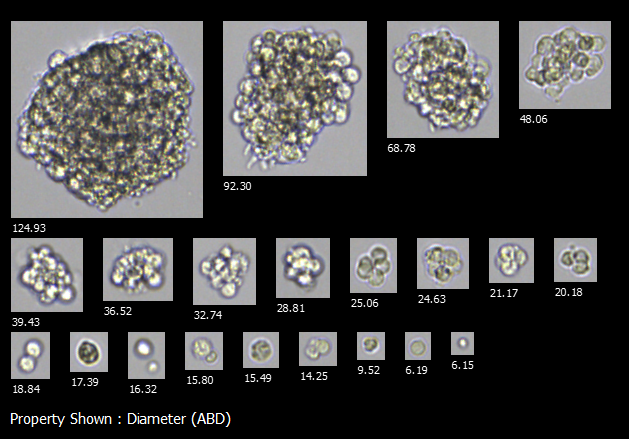
Use FlowCam to monitor how organoid growth conditions influence developmental hallmarks of size and shape
Monitor Cell Populations and Impurities with FlowCam
The inherently large size of therapeutic cells (4-15 µm) restricts the use of sterile filtration and other size-based separations to remove subvisible process impurities and contaminants like DynabeadsTM and cellular debris.
Flow imaging microscopy provides high-quality images to differentiate between particle types including viable and nonviable cells, cell debris, and contaminants. This information enables the removal of unwanted particles at the source – bypassing the need for filtration.
- Differentiate between cells, cell-based impurities, and process-based impurities like Dynabeads to optimize CAR-T and other cell-based drug product manufacturing
- Utilize image analysis tools in VisualSpreadsheet to measure cell concentrations and viability in samples without the need for fluorescence labeling
- Monitor the aggregation of cells into large cell clusters and organoids
Additional Resources
 Guides
Guides
- Advancing Subvisible Particle Analysis: Flow Imaging Microscopy
- Ultimate Guide to Flow Imaging Microscopy for Biotherapeutics
 Blog Posts
Blog Posts
- What are the USP <787>, USP <788>, and USP <789> Standards?
- What are the Recommendations in USP <1787> and <1788>?
- Optimizing Organoid Research with Quality Control of 3D Cell Cultures
- Imaging Organoids with FlowCam - An important step in advancing disease modeling and novel therapeutic drug screening

Interested in learning more?
-
Get in Touch
Tell us about your application and particle characterization needs.
-
Have a Conversation
We're happy to set up a call to discuss your application and answer your questions.
-
Discuss Next Steps
Expand your knowledge with a seminar, demonstration, sample analysis, or obtain a quote.










