Animal testing for every new drug development protocol has been a requirement since the original 1938 passing of the US Federal Food, Drug, and Cosmetic Act. While big questions have been answered and huge gains realized, the 2022 signing of the FDA Modernization Act 2.0 has shifted drug development research away from animal testing towards emerging technologies that promise to efficiently identify safer, more effective drugs and get them to market with substantially lower costs.
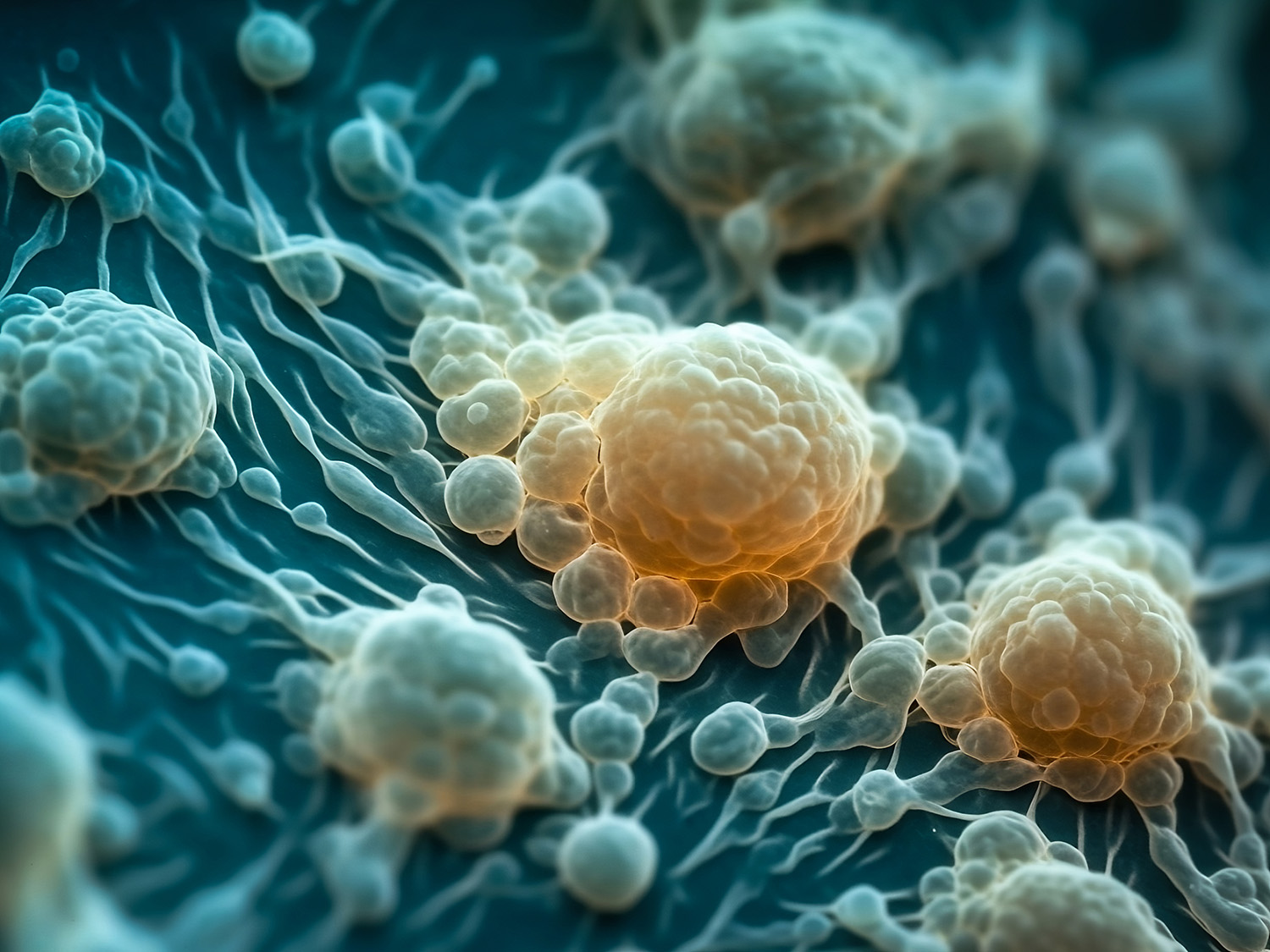
Organoid technology is finding a welcome reception across biomedical research as a swift alternative to whole-animal testing. Scientists are quickly learning how to grow a small mass of cells or tissue into 3D structures that resemble the multicellular organization and functional features of whole organs. Called “organoids,” these miniaturized organs can be maintained under conditions that mimic disease state micro-environments and enable scientists to perform toxicity tests of experimental new drugs in a high-throughput fashion. Organoids grown in bioreactors are maintained in suspension and thus can be monitored in real-time with FlowCam flow imaging microscopy (FIM) to report how growth conditions influence developmental hallmarks of size and shape. An example of organoid imaging by FIM with FlowCam is shown below.

Figure 1. FlowCam images of human kidney organoids at various stages of assembly.
In 2009, Sato et al. described the development of small intestine (crypt-villus) organoids from a single stem cell. They paved the way for the complex multicellular organoid models being studied today. To generate organoids, adult stem cells (ASCs), embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs) are obtained directly from humans or animals and grown in a matrix that is supplemented with proteins and growth factors that guide propagation and targeted differentiation into simplified versions of whole organs. Patient-derived organoids (PDOs) are of special interest in cancer research because they closely mimic the original tumor's architectural and genetic profiles, greatly streamlining drug screening results and treatment prediction accuracies.
One of the greatest challenges organoid technology faces is the development of repeatable, reproducible, and scalable culture protocols. Like most biological interactions, structure begets function, and to realize their potential in the world of precision medicine, manufacturing will need to be tailored for organoids to meet size and shape standards that optimize high throughput drug screening efficiency for novel drug discovery.
Large-scale bioreactors are being well-vetted as the approach for the commercial production of organoids. In contrast to conventional 2D static culture, bioreactors are dynamic 3D culture systems1 where organoids experience environmental conditions that mimic whole organ contexts like fluid flow and nutritional content gradients that positively impact growth and scalability. The use of stirred-tank bioreactors for organoid culture has recently shown2 how improved aeration and distribution of nutrients encourage the formation of complex structures and substantially increase the differentiation yield.

Figure 2: Commonly used bioreactors for organoid culture. (A) Stirred bioreactor with axial impeller (left) and radial impeller (right). Arrows show direction of fluid movement. (B) Microfluidic bioreactor with separate channels for perfusing different media. (C) Rotating wall vessel (RWV) bioreactors: (left panel) Slow Turning Lateral Vessel (STLV) and (right panel) High Aspect Ratio Vessel (HARV). Arrows show direction of rotation. (D) Electrical stimulation bioreactor shows two parallel plate electrodes. Figure adapted from: Licata et al1.
Size, shape, and viability are important features scientists track to assess the physiological relevance of organoid models. Positioning a FlowCam in proximity to bioreactor systems facilitates “at-line” monitoring of organoid suspensions. Samples periodically taken from the bioreactor are directly applied to the instrument for image-based surveillance. High throughput and quantitative reporting of organoid size distributions accurately represent culture growth characteristics in real-time, which can inform workflow protocols related to organoid behavior. In a single sample analysis, high-resolution images of individual organoids combined with quantitative particle property statistics of all organoids imaged in the run reveal a holistic view of organoid development not possible with traditional manual microscopy.
1. Licata JP, Schwab KH, Har-El YE, Gerstenhaber JA, Lelkes PI. Bioreactor Technologies for Enhanced Organoid Culture. Int J Mol Sci. 2023 Jul 13;24(14):11427. https://doi.org/10.3390/ijms241411427. PMID: 37511186; PMCID: PMC10380004.
2. Ovando-Roche, P., West, E.L., Branch, M.J. et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther 9, 156 (2018). https://doi.org/10.1186/s13287-018-0907-0