Cell-based medicinal products (CBMPs) or cell therapies, offer promising opportunities for treating diseases with previously limited or no therapeutic options. However, their complexity and intrinsically fragile nature create significant challenges in formulation development, analytical characterization, manufacturing, and stability assessment.
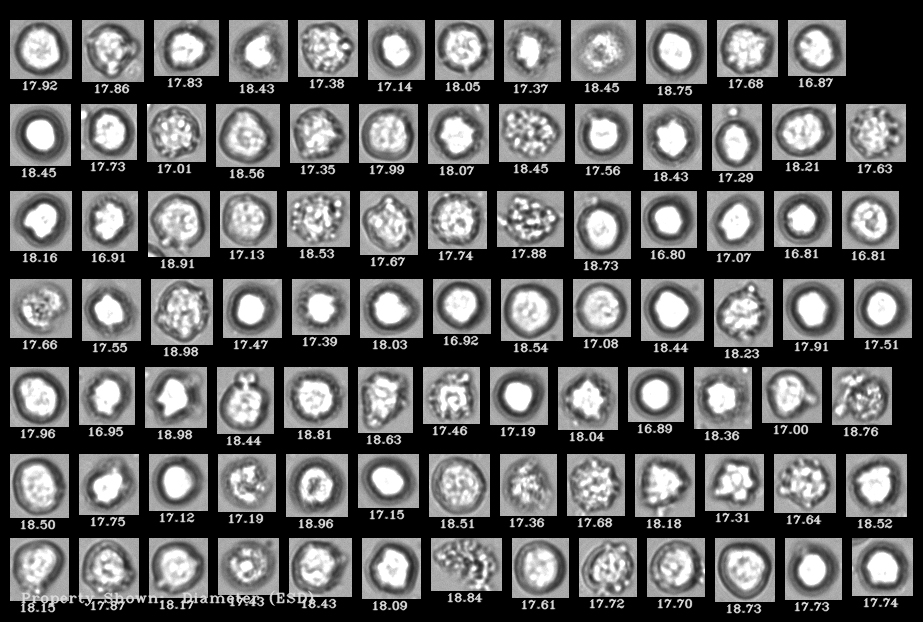
This recent study used FlowCam, a Flow Imaging Microscope (FIM) assisted by machine learning software, to measure cell viability and concentration and to quantify debris particles that occurred as a result of forced degradation studies. These studies mimicked conditions from cell procurement to product administration—in particular, the effects of freeze-thawing and shaking on CBMPs.
Extensive testing of CBMPs before patient administration is a regulatory obligation to assure the safety and efficacy of products. Reliable analytical methods for the characterization of particles to determine product quality and stability are necessary. In this study a FlowCam 8100 (FIM) was combined with convolutional neural networks (FIM-CNN) to establish a high-throughput method to quantify viable and dead cells, and debris particles in the size range of 1-50 µm.
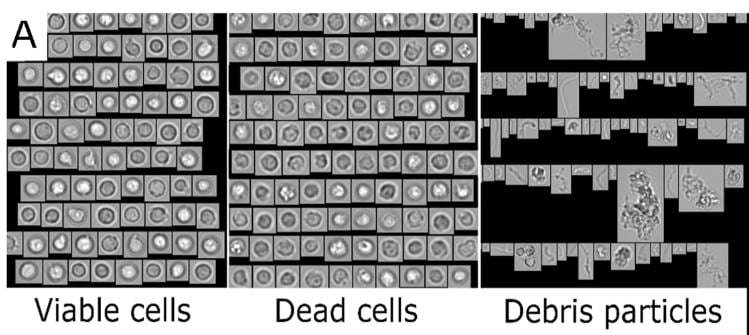
Given the nature of cells, it can be challenging to fulfill certain particle testing requirements for CMBP products. The study notes that light obscuration, one of the standard pharmacopeial methods for quantification of subvisible particles, is not capable of discriminating between cells and other particulates (due to size, shape, and opacity). High-throughput microscopy methods that can provide morphological data are promising tools to evaluate particulates in cell-based products. Using this combination of FIM and CNN, Coriolis was able to obtain reliable concentration data for debris particles and viable cells.
Learn more about FlowCam for analysis of cell therapy products
Reference
A.D. Grabarek, W.Jiskoot, A.Hawe, K.Pike-Overzet, T.Menzen "Forced Degradation of cell-based medicinal products guided by flow imaging microscopy: Explorative studies with Jurkat cells" European Journal of Pharmaceutics and Biopharmaceutics, July 16, 2021 https://doi.org/10.1016/j-ejpb.2021.07.004