Lipid nanoparticles (LNPs) are a type of nanocarrier with immense potential, serving as a tool to deliver nucleic acids like messenger RNA (mRNA) and small interfering RNA (siRNA) to patient cells. They have gained enormous attention as effective drug delivery vehicles for gene therapy, especially since their successful deployment in both the Moderna and Pfizer / BioNTech SARS-CoV-2 vaccines. Each vaccine deploys LNPs to protect a mRNA payload from degradation in the body and safely deliver the nucleic acid to the cytosol of target cells.
Despite their burgeoning success, developing stable lipid nanoparticle formulations that minimize aggregation and fusion remains challenging. These issues can lead to reduced transfection efficiency and other unwanted changes in product efficacy. Therefore, understanding and controlling LNP aggregates is essential to maximize the efficacy of LNP therapies for patients.
Download the Application Note: FIM to Monitor LNP Aggregation
What are Lipid Nanoparticles?
Lipid nanoparticles are spherical assemblies of lipid molecules designed to encapsulate nucleic acids. Several lipid types are used to synthesize LNPs, including ionizable lipids, “helper” phospholipids, cholesterols, and polyethylene glycol-lipids (PEG-lipids). Each lipid type plays a different role in LNP function. Ionizable lipids exhibit an amphiphilic structure that can become charged depending on the pH of the environment. Their pH-dependent charge plays a critical role in encapsulating the RNA payload, protecting the nucleic acid in the body, and facilitating endosomal escape of the RNA in the target cell. Phospholipids improve the overall stability of the LNP particle, helping stabilize lipid bilayers and improving RNA encapsulation. Cholesterol is used to control the fluidity of lipid membranes in the LNP. PEG-lipids are used to mitigate LNP aggregation as well as increase the circulation time of the LNP in the body.
LNPs can be synthesized from the component lipids and RNA payload via several methods. In one common method, an ethanol-based solution containing the lipids is mixed with an aqueous solution at an acidic pH containing the RNA payload. The mixing can be performed manually or automatically using microfluidic devices. The lipids become less soluble and charged upon mixing, causing them to self-assemble into LNPs and encapsulate the RNA molecules.
Lipid nanoparticles are designed to be robust to aggregation, particularly through the use of PEG-lipids. Despite this design, LNPs are still capable of forming aggregates due to conditions the drug substance is exposed to in their formulation and manufacturing process. Let’s explore some of the factors that influence aggregation.
Factors Influencing LNP Aggregation
The types of lipids used in LNP formulations significantly influence their stability and propensity to aggregate. PEG-lipids are the primary lipid used to control aggregation as PEG creates a steric barrier to aggregation during storage. It is worth noting that PEG-lipids inhibit but not prohibit lipid nanoparticle aggregation. LNPs containing PEG-lipids can still form aggregates, especially under the environmental conditions discussed later in this section. Additionally, PEG-lipids may lead to reduced cellular uptake and endosomal escape. They may also contribute to the formation of anti-PEG antibodies which may cause hypersensitivity reactions that are observed (albeit infrequently) with LNP therapies. It is, therefore, essential to optimize the amount of PEG-lipid used in LNPs and investigate additional and alternative strategies for preventing LNP aggregation to reduce the amount of PEG-lipid required for stability.
The structure of the other lipid types can also influence the likelihood of lipid nanoparticle aggregation. For example, some ionizable lipids like those in the SARS-CoV-2 vaccines have hydrophobic tails with a pronounced branching structure. These branched tails result in LNPs with more rigid membranes, which helps reduce aggregation.
Environmental Conditions
Many conditions that LNP drug products may be exposed to before use can promote the formation of lipid nanoparticle aggregates. Common stresses that may lead to aggregation include exposure to elevated temperatures, mechanical agitation, and freeze-thaw cycles. For example, a recent study found that even slight mechanical agitation when administering an LNP therapy can cause the formation of LNP particles1. Other common stresses that lead to aggregation include exposure to elevated temperatures and freeze-thaw cycles.2,3 The latter can be problematic for drug products designed to be stored frozen or lyophilized (i.e., freeze-dried), as they must be exposed to at least one freeze-thaw cycle before use. Designing lipid nanoparticles and their formulation to stabilize the LNP when exposed to stresses the sample may encounter in manufacturing, shipping, and storage is crucial.
LNP Formulation
Several formulation excipients can influence the extent of lipid nanoparticle aggregation in a sample. Formulation pH can influence LNP fusion and aggregation, with aggregation occurring more rapidly at neutral pH, where ionic lipids are close to neutrally charged. Similarly, higher ionic strength formulations also promote aggregation due to greater charge screening between LNP particles.
Many LNP drug products are stored frozen or lyophilized, so protecting them from aggregation induced by freeze-thaw cycles is important. Cryoprotectants such as trehalose and sucrose help minimize LNP aggregation from freeze-thaw cycles. The choice of buffer is also crucial for frozen LNP drug products. Some buffers, like phosphate-buffered saline (PBS), can undergo significant changes in pH during freezing and thawing, potentially inducing further aggregation.
Irrespective of the storage condition, the formulation of LNPs with appropriate excipients is crucial to minimize the extent of aggregation in LNP drug products. This underscores the need for effective LNP formulations to ensure the stability of these therapies.
Exploring Lipid Nanoparticle Formulation Stability
Given these issues, monitoring LNP formulations for aggregates and other larger lipid assemblies is essential. Flow imaging microscopy (FIM) instruments such as the FlowCam 8000 series and FlowCam Nano can be used to monitor drug products for LNP-derived particles and other unwanted subvisible particles. Their measurements can help scientists monitor and control lipid nanoparticle aggregation and improve the overall stability of LNP therapies.
We conducted a study demonstrating how flow imaging microscopy can be used to analyze aggregation and particle formation in lipid nanoparticle formulations. Aliquots of an LNP formulation were exposed to either accelerated freeze-thaw stress or accelerated heat stress to induce aggregation. Using a FlowCam 8000 series instrument and FlowCam Nano, we analyzed the stressed samples' particle concentrations, sizes, and images. These FIM measurements revealed that the heat stress generated higher concentrations of subvisible particles (i.e., those 2-100 µm in diameter), indicating that heat stress had a greater negative impact on LNP stability for this formulation. Protecting the LNP against heat stress through formulation design or process improvements would improve the overall stability of the drug product.
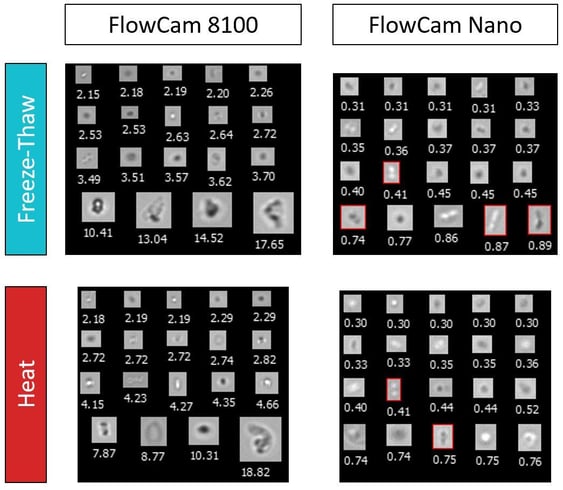
This figure includes images captured using FIM, depicting particles from freeze-thaw- and heat-stressed samples. Using FlowCam Nano, we were able to detect small lipid nanoparticle aggregates. For example, the boxed images exhibit a dimer-like structure that may have been generated via aggregation. These high-resolution, single-particle precision images allow LNP aggregation to be detected at its onset, allowing researchers to proactively identify and mitigate aggregation in these therapies.
 Understanding and controlling lipid nanoparticle aggregation is paramount for researchers to realize the full potential of lipid nanoparticle therapies. FlowCam instruments enable scientists to effectively monitor LNP aggregation—even at the earliest stages of oligomerization.
Understanding and controlling lipid nanoparticle aggregation is paramount for researchers to realize the full potential of lipid nanoparticle therapies. FlowCam instruments enable scientists to effectively monitor LNP aggregation—even at the earliest stages of oligomerization.
For more information, download the application note to read the complete study:
References:
- Hada S, Na KJ, Jeong J, Choi DH, Kim NA, Jeong SH. Evaluation of subvisible particles in human immunoglobulin and lipid nanoparticles repackaged from a multi-dose vial using plastic syringes. Int J Biol Macromol. 2023;232(September 2022):123439. doi:10.1016/j.ijbiomac.2023.123439
- Ball RL, Bajaj P, Whitehead KA. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int J Nanomedicine. 2017;12:305-315. doi:10.2147/IJN.S123062
- Reinhart AG, Osterwald A, Ringler P, et al. Investigations into mRNA Lipid Nanoparticles Shelf-Life Stability under Nonfrozen Conditions. Mol Pharm. 2023;20(12):6492-6503. doi:10.1021/acs.molpharmaceut.3c00956