Pharmaceutical researchers face mounting pressure to track and control particles in their drug formulations throughout development. Strategies to monitor subvisible particles (those 2-100 μm in diameter) are now critical to formulation design. Traditional particle analysis techniques like light obscuration (LO) and membrane microscopy are limited in their ability to track common particle types in modern pharmaceuticals. This underscores the need for more advanced technologies, such as flow imaging microscopy (FIM), that allow for more precise particle analysis than these methods, improving drug formulation quality.

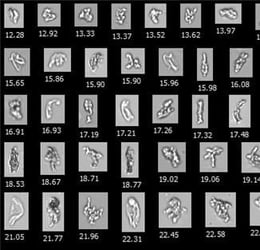
Pictured above: A National Institute of Standards and Technology protein standard, a commonly used reference material in pharmaceutical research, imaged by FlowCam. This image demonstrates the high-resolution capabilities of FIM and its potential in drug formulation analysis.
Ensuring Quality Standards and Compliance
Particle concentrations and size distributions are critical quality attributes for many pharmaceuticals and are thus essential to monitor in formulation development as part of quality assurance. Particles have been associated with adverse reactions in the clinic, ranging from capillary occlusion to antidrug antibody formation and anaphylaxis. Pharmacopeia requirements such as USP <788> also set limits on particulates in parenteral drug products. Flow imaging microscopy provides formulation scientists with accurate particle measurements that they can use during drug formulation design to create stable, high-quality formulations that maximize product safety and ensure compliance with regulatory guidelines.
The Power of Flow Imaging Microscopy
Flow Imaging Microscopy (FIM) via FlowCam uses brightfield microscopy principles coupled with microfluidics to capture high-resolution images of each particle in a drug formulation. These images allow for accurate particle count and size measurements over a wide size range, including subvisible and visible particles between 2 and 1000 μm in diameter. The images also contain significant particle morphology information, allowing precise particle characterization. These concentration, size, and morphology measurements enable better control of the particle content within drug formulations, improving the quality of the final drug product.
Identifying Particle Sources During Drug Formulation Development
Modern drug products, especially biotherapeutics, often have complex drug formulations. The drug substance, formulation excipients, and container-closure system in a formulation can each contribute particles. Understanding which components generate particles is crucial in formulation design to minimize particle content. The morphology data FIM provides indicates particle origin and empowers formulation scientists to make targeted formulation changes to eliminate unwanted particles at the source.
To optimize the subvisible particle content in drug formulation development, researchers often need to measure several attributes of the particles in a sample. Particle concentration and size distribution data allow researchers to compare particle levels quantitatively between formulations. Measurements that provide information on particle type, such as morphology or composition, can then be used to determine the sources of those particles—data that can guide further changes to the formulation to reduce particle concentrations.
Historically, researchers have had access to several techniques for measuring subvisible particles in candidate formulations. However, many of these techniques only provide some of the information about particles necessary to inform formulation decisions. Particle counters like light obscuration (LO) provide accurate particle concentration and size data but do not capture information about the types and sources of particles in a sample. Microscopy methods like membrane microscopy capture morphology information but offer less representative particle concentration and size distribution measurements due to their low throughput and often invasive sample preparation.
Flow Imaging Microscopy in Biotherapeutic Development
Controlling particulate matter is particularly important in biotherapeutic formulation development. All biotherapeutic formulations contain subvisible particles, many of which are inherent particles or those derived from the drug substance. Identifying inherent particle types like protein aggregates in a sample is critical as they are thought to pose significant risks to patient safety. However, these particles can be difficult to count and size accurately due to their translucent, often fragile structures. Despite these challenges, FIM offers sensitive measurements of inherent particles and provides morphology information that can distinguish them from intrinsic particles derived from other drug product components. For example, FlowCam's VisualAITM software allows researchers to automatically distinguish protein aggregates from silicone oil droplets using FlowCam images, streamlining protein formulation development.
The Role of Flow Imaging Microscopy in Particle Analysis
As noted, FIM technology provides insight into drug formulations' particle concentration, size, and morphology. It is beneficial for identifying problematic particles like silicone oil droplets, glass lamella, and free fatty acid particles. Characterizing these particles during formulation design is critical in identifying excipients and container-closure systems that stabilize the drug substance and minimize other particle sources. This quality-by-design approach for particle control helps researchers meet pharmacopeia standards. It is recommended via USP <1788> as an orthogonal technique to light obscuration and membrane microscopy to characterize subvisible particles - especially during development. These efforts also minimize the risks particles pose to patients, improving product safety and efficacy.
Interested in FIM with FlowCam?
Flow imaging microscopy has become a cornerstone of modern quality assurance in drug product development. Its accurate particle concentration, size distribution, and morphology measurements ensure that manufacturers meet the highest quality standards while safeguarding patient health. For drug manufacturers, adopting FIM with FlowCam can be key to maintaining product integrity, ensuring regulatory compliance, and advancing therapeutic innovation.