Biopharma
FlowCam for Protein Aggregation Analysis in Therapeutics Development
Proteins can aggregate together in solution, creating particulates in the drug product that are associated with adverse reactions to the therapy. These reactions can hinder the success of the therapy in the clinic, resulting in failed drug products and product recalls.
FlowCam uses high-throughput flow imaging microscopy to capture high-resolution images of protein aggregates and other particulates in protein, monoclonal antibody, or antibody-drug conjugate formulations. As each particle flows through the imaging system, it’s photographed and measured, enabling scientists to distinguish protein aggregates from silicone oil, degraded polysorbate, glass particles, and other contaminants based on size, shape, and morphology.
This level of insight provides the precise, visual confirmation needed to manage development controls and support the formulation process.

Detect Protein Aggregates in Parenterals
Image-based analysis helps formulation teams:
- Count and size protein aggregates as small as 300 nm with industry-leading image quality
- Obtain complementary particle image data recommended by USP <1788> to verify orthogonal particle size measurements by light obscuration
- Utilize image-based analytics including artificial intelligence tools to classify subvisible and submicron particles
Helpful Resources
“[FlowCam] generated very valuable data for our team much faster than traditional membrane microscopy methods, and I like that it's both a quantitative and qualitative method in one.”

A FlowCam collage of common particles in therapeutic protein formulations including protein aggregates, silicone oil droplets, and polysorbate particles.

The FlowCam family of products for biopharmaceutical applications: FlowCam 8100, FlowCam Nano, FlowCam LO
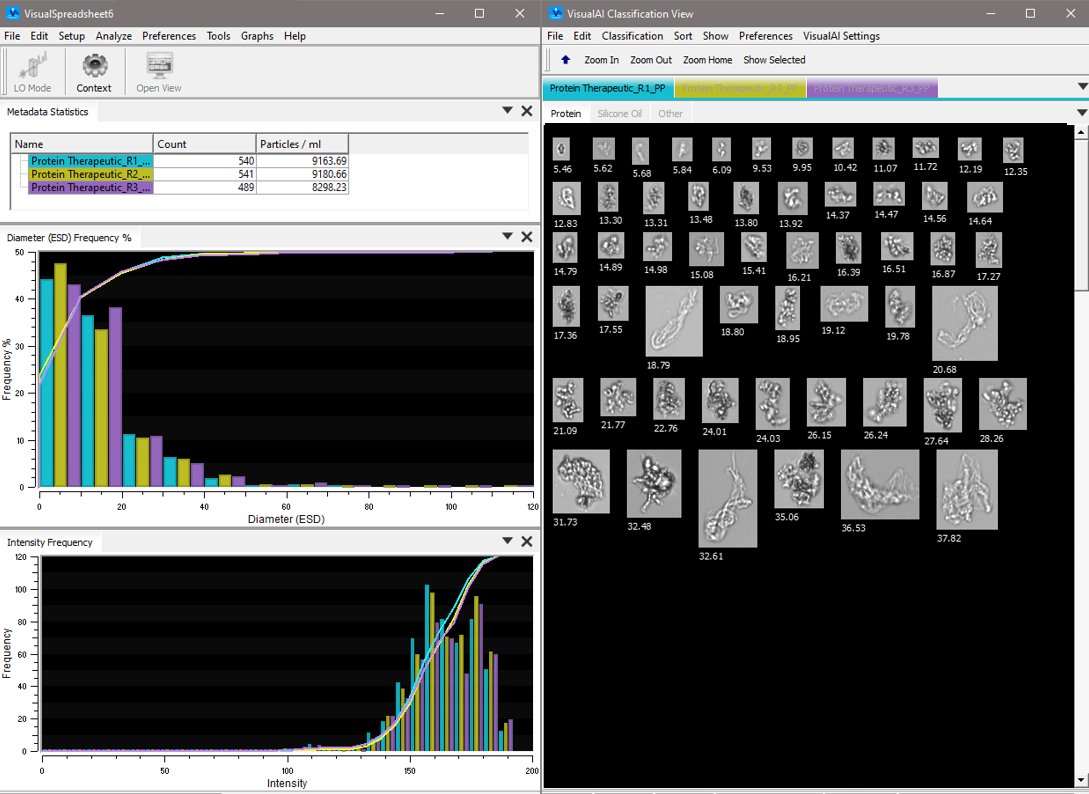
VisualSpreadsheet software interface showing protein aggregates automatically identified with VisualAI artificial intelligence.
How FlowCam Produces More Accurate Protein Aggregate Analysis
Capturing high-resolution images in real time enables FlowCam to deliver fast, precise identification and differentiation of protein aggregates and other particles.
This accuracy helps scientists detect and mitigate undesirable or potentially harmful aggregates early in the formulation development process, improving safety and reducing risk at the source.
Key features include:
- USP <787> compendial particle sizing and imaging in a single instrument with FlowCam LO.
- Automated Liquid Handling (ALH) integration for highly productive and reproducible sample processing.
- VisualAI™ technology that uses artificial intelligence to automatically classify images of protein biotherapeutics with a higher than 90% accuracy.
Learn More About Protein Aggregate Analysis
-
Protein aggregate analysis involves detecting, sizing, and characterizing protein particles that form when therapeutic proteins degrade. This analysis is critical because protein aggregates pose significant risks for patient safety, potentially leading to treatment failure and other dangerous adverse reactions.
-
Several analytical techniques are available for characterizing protein aggregates, each offering different types of data and suited to various stages of development:
- Light Obscuration: A common quality control method that counts and sizes particles typically above 1 µm based on light blockage. While fast and automated, it provides no visual information to distinguish particle types, potentially leading to inaccurate classification of contaminants.
- Membrane Microscopy: Involves visual inspection of particles using a standard brightfield microscope after using membrane filtration to remove the formulation buffer. It provides qualitative morphological information but is labor-intensive and can bias the number, sizes, and types of particles detected.
- Dynamic Light Scattering (DLS): Estimates nanoparticle size distribution in solution by measuring fluctuations in scattered light intensity. DLS is rapid and sensitive, but it struggles with samples containing a polydisperse particle population, especially those with a high concentration of subvisible particles. It also does not distinguish between different particle types in a sample.
- Multi-Angle Light Scattering (MALS): Often used as a detector for methods like size-exclusion chromatography and field-flow fractionation. MALS provides detailed molecular weight and size information for monomeric proteins and small, "soluble" protein aggregates. However, protein formulations can exhibit significant differences in subvisible and visible protein aggregate content even with very little changes in this size range.
- Flow Imaging Microscopy: Integrates microscopy with continuous flow analysis, capturing high-resolution images of thousands of particles per minute as they flow through the instrument. This approach balances the speed of light obscuration with the detailed imaging of manual microscopy for effective, accurate analysis.
-
FlowCam flow imaging microscopes can detect and image particles from 300 nm to 1 mm, depending on the selected instrument model. This broad detection capability makes FlowCam ideal for visualizing and quantifying particles across both subvisible and visible size ranges, as well as submicron sizes.
-
FlowCam instruments support USP <788>, <787>, <789>, and even <790> compliance by offering more sensitive detection of protein aggregates and other sources of particulate matter than the compendial methods. Minimizing the particle content of a protein drug product measured by FlowCam improves the likelihood of that therapy meeting USP requirements when measured via the compendial methods. Additionally, when a drug product fails to meet USP requirements, FlowCam provides particle morphology information that can determine and resolve the source of any unexpected particles.
Flow imaging microscopy is a recommended orthogonal technique for subvisible particle monitoring via USP <1788>. With FlowCam LO, it is even possible to capture FIM data alongside compendial light obscuration measurements, streamlining subvisible particle monitoring to USP <1788> recommendations.
-
FlowCam is particularly helpful in the later stages of formulation design, when comprehensive, quantitative particle characterization becomes critical. Its measurements are used to quantify the stability of protein drug products during accelerated, real-time, and in-use stability studies. It is also often used as a secondary analytical tool in quality control testing, complementing primary lot release tests to diagnose unexpected changes in particle content and ensure that each batch contains a consistent, safe amount of particles.
-
Key features of protein aggregate particles include their shape and coloration. Protein aggregates typically appear as irregular, somewhat transparent particles. In contrast, silicone oil droplets appear as perfectly spherical droplets with more pronounced coloration.
FlowCam's VisualAI™ software can automatically classify these particle types with over 90% accuracy, reducing subjectivity in particle identification.
-
FlowCam can be used independently or as an orthogonal or complementary analytical method to provide more complete characterization of the particles in a sample. It provides labs with important information about the size, shape, and type of protein aggregates and contaminants, complementing techniques that measure other aspects, such as molecular weight or concentration. Together, these tools give a fuller picture of a formulation’s stability and quality.
Additional Resources
 Guides
Guides
- Advancing Subvisible Particle Analysis: Flow Imaging Microscopy
- Ultimate Guide to Flow Imaging Microscopy for Biotherapeutics
 Blog Posts
Blog Posts
- What are the USP <787>, USP <788>, and USP <789> Standards?
- What are the Recommendations in USP <1787> and <1788>?
- What Does "Orthogonal Method" Mean for Particle Analysis?
- What's the Value of Monitoring Silicone Oil Droplets in Protein Therapeutics?
 Videos
Videos
 Application Notes
Application Notes
 Published Research
Published Research

Interested in learning more?
-
Get in Touch
Tell us about your application and particle characterization needs.
-
Have a Conversation
We're happy to set up a call to discuss your application and answer your questions.
-
Discuss Next Steps
Expand your knowledge with a seminar, demonstration, sample analysis, or obtain a quote.










