Comprehensive characterization of biotherapeutics is often challenging, given their complexity. In many cases, singular analytical methods cannot provide the complete picture of specific attributes. Orthogonal methods—different methods intended to measure the same attributes—are often necessary to provide independent confirmation of Critical Quality Attributes (CQAs). CQAs are essential characteristics that must fall within a specific range to guarantee the quality of a drug product1. Measuring these attributes accurately is essential to obtain accurate shelf life estimates and reliable clinical data during development. Accurate CQA data during manufacturing is needed to ensure batch consistency and adherence to release specifications and demonstrate product comparability following manufacturing changes.
The terms “orthogonal” and “complementary” are often used to describe the relationship between different techniques used to characterize a therapeutic sample2. These relationships determine the information we gain about a sample’s attributes from combining analytical methods. This blog post will introduce these terms and provide examples of both types of measurements for monitoring particles in biopharmaceuticals using flow imaging microscopy (FIM).
Download the White Paper: "Orthogonal and Complementary Techniques"
What are Orthogonal Methods?
Orthogonal methods capture information about the same sample attribute using different measurement principles. They are often used to obtain a more accurate description of a single, important property. All analytical techniques have a bias or systematic error that arises from their measurement principle and any necessary sample preparation. Scientists can use orthogonal techniques to obtain multiple values of a single CQA biased in different ways, which can be compared to control for the error of each analysis.
Many analytical methods have a dynamic range that limits the species in a drug product that they can analyze. For example, many particle monitoring strategies will only analyze particles in a specific size range, such as nanoparticles (<1 μm in diameter) or subvisible particles (2-100 μm in diameter). Orthogonal techniques must also provide measurements over the same dynamic range.
What are Complementary Methods?
Complementary methods are any techniques that provide additional information about relevant sample attributes for describing a sample2. This includes analyses that provide information about different CQAs (e.g., pH and tonicity) as well as methods that analyze the same nominal property but over a different dynamic range (e.g., nanoparticle size distribution and subvisible particle size distribution). By this definition, all orthogonal measurements are also considered complementary as they provide additional information about a single feature of a sample. However, in common usage, “complementary” refers to techniques that are not orthogonal and, therefore, provide information on different sample attributes. Complementary but not orthogonal measurements are essential in monitoring all relevant features for a specific sample.
Some instruments provide information about multiple sample properties, such as particle size and concentration. When comparing a pair of analytical techniques that analyze multiple CQAs, some properties may be measured by both methods, and others may only be analyzed using one approach. It is, therefore, possible for two measurements to be orthogonal, complementary, or even both, depending on which quality attributes are relevant to a specific sample and research question.
Orthogonal and Complementary Techniques for Particle Size Analysis
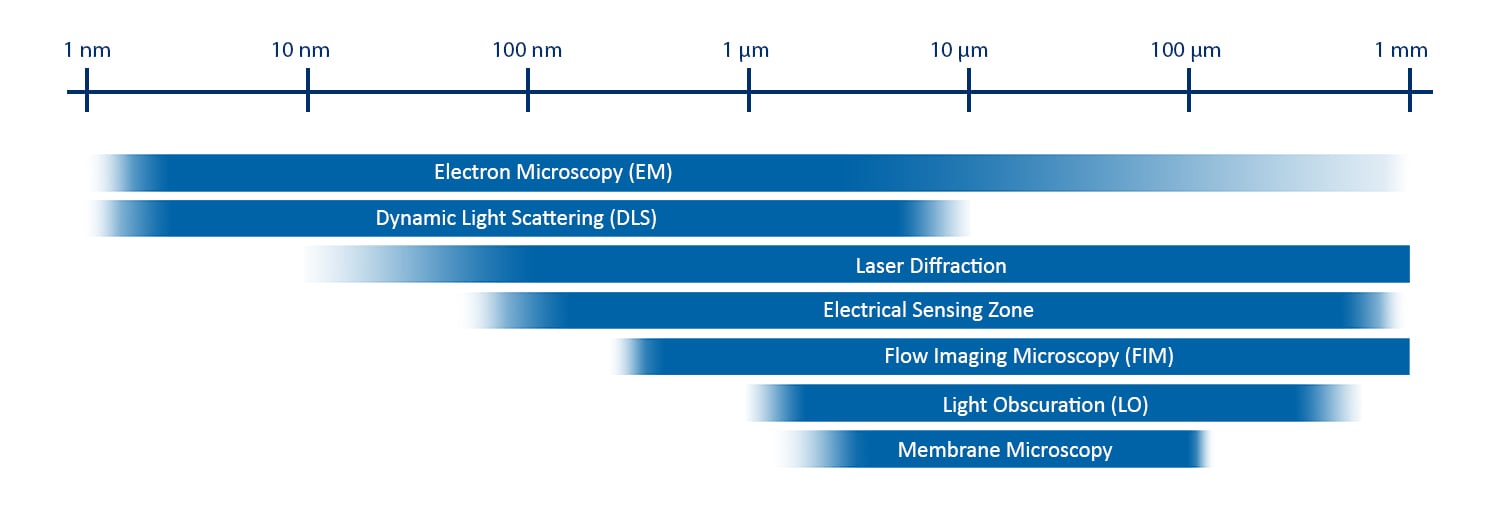
Pictured above: common analytical techniques used to monitor particle size. The position and length of each bar indicate the dynamic range of that method.
To provide examples of both method types, let’s assume that we are interested in characterizing a biopharmaceutical sample and want to use particle size distribution as one of our critical quality attributes. We will also assume that we want to use flow imaging microscopy (FIM) as one of our analytical techniques. FIM is often used to capture information about the particle size distribution in the subvisible (2-100 μm) range as well as monitor particle concentration and morphology. What other measurements could be used to capture information about this sample?
Orthogonal techniques to flow imaging microscopy
A common orthogonal method to FIM is light obscuration (LO), which provides an additional reading of the size distribution and concentration of subvisible particles. LO and FIM use distinct measurement principles to capture this information—light blockage and digital imaging, respectively. FIM has been shown to size and count many common particle types in biopharmaceutical samples, such as protein aggregates, more accurately than LO3. Conversely, LO data often needs to be captured to ensure compliance with pharmacopeia guidelines on subvisible particles like USP <788>. Capturing both orthogonal measurements allows scientists to get accurate particle size data in the subvisible range while checking for compliance with pharmacopeia limits.
FlowCam LO simplifies performing these orthogonal methods. This instrument can provide FIM and LO data using a single sample aliquot, greatly reducing the time, effort, and sample volume required to obtain this information. Capturing both measurements with a single instrument is especially ideal for bridging studies to compare FIM against legacy LO data.
Complementary techniques to flow imaging microscopy
Researchers have several options available for complementary methods to FIM, depending on the information they need to collect for a given therapy. One option is to select techniques that monitor a completely different CQA. Some options include circular dichroism to monitor protein conformation in a protein therapy or analytical ultracentrifugation to measure full/empty ratios for an AAV therapy. These measurements are complementary to FIM as they record information about a different, albeit relevant, critical quality attribute.
One could also use techniques that measure particle size over a different size range. Common examples include dynamic light scattering and size exclusion chromatography, both of which analyze nanoparticle size distributions. These methods are complementary to FIM since they analyze the same attribute but over a different dynamic range.
Orthogonal and complementary measurements are essential in obtaining an accurate and complete understanding of a pharmaceutical sample. For more information on these terms and to learn about different orthogonal and complementary methods for particle analysis, download our white paper "Orthogonal and Complementary Techniques: Combining Flow Imaging Microscopy with Light Obscuration to Characterize Biotherapeutics."
References
- International Conference on Harmonization. ICH Q8 (R2): Pharmaceutical Development. Int Conf Harmon Tech Requir Regist Pharm Hum Use. 2009;8(August):28. https://www.ich.org/page/quality-guidelines
- Simon CG, Borgos SE, Calzolai L, et al. Orthogonal and complementary measurements of properties of drug products containing nanomaterials. J Control Release. 2023;354:120-127. doi:10.1016/j.jconrel.2022.12.049
- Shibata H, Harazono A, Kiyoshi M, Ishii-Watabe A. Quantitative Evaluation of Insoluble Particulate Matters in Therapeutic Protein Injections Using Light Obscuration and Flow Imaging Methods. J Pharm Sci. 2021;000. doi:10.1016/j.xphs.2021.09.047