Light obscuration (LO) is the compendial method for quantifying subvisible particles (SVPs) in injectable drugs, harmonized across the US Pharmacopeia, European Pharmacopeia, and Japanese Pharmacopiea. However, multiple studies have shown that "the capability of the LO method is not enough to detect SVPs with low optical contrast"1.
Research has shown Flow Imaging Microscopy (FIM) to be a "powerful tool instead of LO due to the fact that (1) protein aggregates contain highly transparent particles and thereby escape detection by LO, and (2) FIM provides detailed morphological characteristics of SVPs1". In order for FIM to become a compendial method, it must be standardized. In two recent studies, a consortium of Japanese laboratories assessed the standardization of FIM.
In their most recent publication, Quantitative Evaluation of Insoluble Particulate Matters in Therapeutic Protein Injections Using Light Obscuration and Flow Imaging Methods, Shibata et. al. compare the ability of Light Obscuration and Flow Imaging to detect and accurately characterize subvisible particles in injectable drugs.
The team at Japan's National Institute of Health Sciences write the following:
"Flow imaging (FI) has emerged as a powerful tool to evaluate insoluble particles derived from protein aggregates as an orthogonal method to light obscuration (LO). However, few reports directly compare the FI and LO method in the size and number of protein particles in commercially available therapeutic protein injections. In this study, we measured the number of insoluble particles in several therapeutic protein injections using both FI and LO, and characterized these particles to compare the analytical performance of the methods. The particle counts measured using FI were much higher than those measured using LO, and the difference depended on the products or features of particles. Some products contained a large number of transparent and elongated particles, which could escape detection using LO. Our results also suggested that the LO method underestimates the size and number of silicone oil droplets in prefilled syringe products compared to the FI method. The count of particles ≥10mm in size in one product measured using FI exceeded the criteria (6000 counts per container) defined in the compendial particulate matter test using the LO method. Thus precaution should be taken when setting the acceptance criteria of specification tests using the FI method."2
|
|
|
|
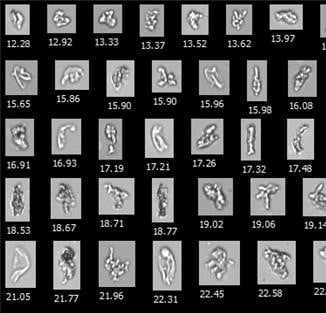
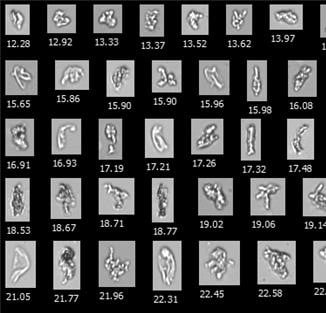
Representative FlowCam images of |
Representative FlowCam images of |
The results of this study corroborate the results produced in our FlowCam lab, comparing light obscuration particle counts and sizes with flow imaging data using our instrument, FlowCam LO.
Our white paper, "Measuring Subvisible Particles and Aggregates Using FlowCam LO", uses FlowCam LO to directly compare the particle size distributions of aqueous samples containing Polystyrene Latex calibration beads, ETFE particles, and IgG aggregates. Download our white paper to see the results of the study.
FlowCam LO uses two orthogonal techniques in a single instrument by combining our patented flow imaging microscopy technology with an embedded light obscuration particle counter. FlowCam LO provides an even more direct particle count comparison because a single aliquot of sample is analyzed by both technologies in one sample run, instead of two samples from the same vial or syringe.
References
- Kiyoshi M, et al. Collaborative Study for Analysis of Subvisible Particles Using Flow Imaging and Light Obscuration: Experiences in Japanese Biopharmaceutical Consortium. Journal of Pharmaceutical Sciences. 2019;108(2);832-841. doi:10.1016/j.xphs.2018.08.006
- Shibata H, et al. Quantitative Evaluation of Insoluble Particulate Matters in Therapeutic Protein Injections Using Light Obscuration and Flow Imaging Methods. Journal of Pharmaceutical Sciences. 2022;111(3);648-654. doi:10.1016/j.xphs.2021.09.047