Proteinaceous particles in parenteral drugs pose an immunogenic risk. These formulations are therefore rigorously characterized for optimal conformational and colloidal stability of the drug molecule. As such, they undergo thorough analysis of biophysical descriptors and extended particle characterization to ensure a safe and stable product is delivered to market with a shelf life of about two years. In this post, we summarize a recent paper by Mattison et al. (2018) published in BioProcess International on how they successfully reformulated biotherapeutics by using quantitative stability predictors and descriptors.

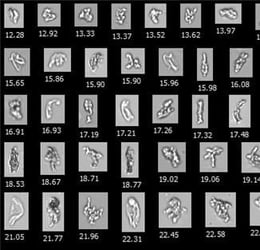
![]() Mattison et al. sorted images based on morphology to distinguish between silicone oil droplets (pre-filled syringe lubricant) and proteins. Credit: Mattison et al., 2018.
Mattison et al. sorted images based on morphology to distinguish between silicone oil droplets (pre-filled syringe lubricant) and proteins. Credit: Mattison et al., 2018.
Different methodologies are used to characterize the liquid protein formulations to measure biophysical descriptors and perform extended particle characterization. An article by Mattison et al. (2018) describes how they rationally achieved the reformulation of a biotherapeutic product based on biophysical stability predictors and descriptors as measured by the initial product ("formulation A") and the reformulated product ("formulation B").
Such predictors and descriptors included:
- diffusion interaction parameter (kD) as measured by dynamic light scattering,
- second virial coefficient (B22) as measured by static light scattering,
- effective protein charge (ZEff), and
- melting and onset aggregation temperatures (TM and TOnset).
The formulations were also characterized using flow imaging analysis via FlowCam to quantify subvisible particle count and morphology. Additionally, the FlowCam was used to sort images based on morphology, enabling the differentiation and separate analysis of amorphous, proteinaceous particles, from round silicone oil droplets used in the prefilled syringe lubricant.
To learn about their method for liquid protein formulation characterization, you can access the full article here.
Citation:
Mattison, K., Mehtala, J., Taddei, M., Cheung, J., Gutka, H. 2018. Rational Design of Liquid Protein Formulations: Application of Biophysical Stability Predictors and Descriptors to Reformulate Biotherapeutics. BioProcess International, 16(5) pp. 32-42.