Beyond Particle Counting: Verifying Particle Identity
Your current particle analyzer counts the subvisible particles in your protein therapeutic. A particle size distribution curve confirms compliance with USP <788> and <787> standards: no more than 6,000 particles ≥ 10 µm and 600 particles ≥ 25 µm. But how do you ensure these detected particles are your intended product and not contaminants or extrinsic, intrinsic, or inherent particles?
The Advantages of Flow Imaging Microscopy
Flow imaging microscopy (FIM) is a recommended orthogonal technique per USP <1787> and <1788>, complementing the two compendial methods—especially during development. These chapters emphasize the value of particle morphology data provided by FIM in identifying particle types. Unlike light obscuration (LO), FIM offers greater sensitivity to protein aggregates and other transparent particle types.
As a non-compendial technique, FIM is often paired with LO to capture morphology data unavailable from LO while still meeting pharmacopeia standards. FlowCam LO seamlessly integrates FIM and LO measurements into a single instrument without requiring additional sample volume. This integration simplifies the inclusion of FIM into lot release processes, enabling researchers to identify unwanted particle sources and potential process upsets before they escalate to batch rejections or compromised product efficacy.
Classifying Particulates Using Morphology
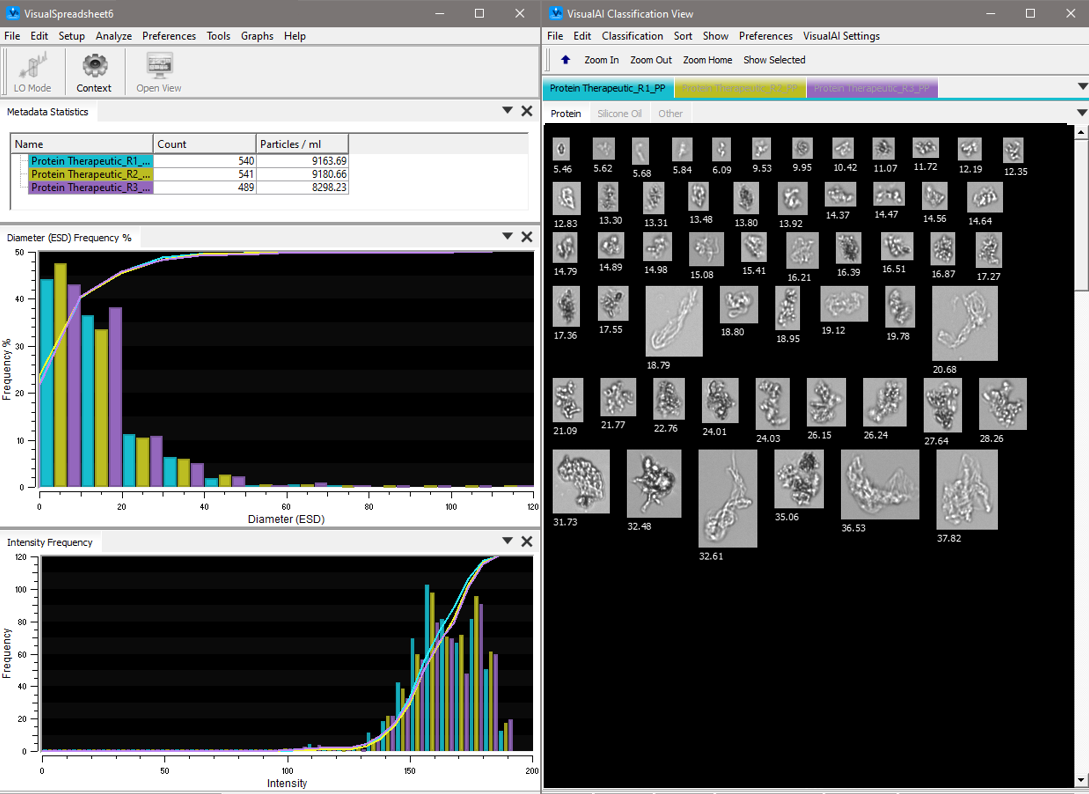
In a study conducted in the FlowCam applications lab, polyclonal IgG from a lubricated, prefilled syringe was analyzed using a FlowCam 8100 instrument and VisualSpreadsheet software. Each subvisible particle was imaged, and over 40 morphological characteristics, including size, were measured. VisualSpreadsheet’s morphological filtering capabilities were used to differentiate silicone oil droplets from other particulates in the sample.
Key parameters such as aspect ratio, circularity (Hu), and statistical filters were combined to create an effective filter. This analysis revealed that 26% of the sample’s particulates consisted of silicone oil. With this information, researchers could perform further morphological and statistical analyses on this population. Additional filters can be created to distinguish and analyze other particulate populations based on size ranges and morphology.
Improved Analyses, Safer Injectables
Protein aggregates are ubiquitous in protein-based drug products and are associated with anti-drug antibody formation and adverse clinical reactions. FlowCam enables researchers to differentiate protein aggregates from other subvisible particles such as silicone oil, degraded polysorbate, and glass flakes (example FlowCam images of these particles are shown below). It also distinguishes between protein aggregates originating from different sources, helping users identify and mitigate manufacturing, shipping, storage, and usage stresses.
![]()
By analyzing particles based on shape rather than size alone, FlowCam provides the critical insights needed to develop safe and effective biotherapeutics. This capability enhances your ability to ensure product quality and patient safety, making it an indispensable tool in the development and production of injectable protein therapeutics.
Learn more about the advantages of morphological analysis in our ebook, The Ultimate Guide to Flow Imaging Microscopy.