Optimizing FlowCam context settings and instrument parameters used during particle analysis is an important step in developing a protocol for collecting FlowCam data. Capture settings such as the segmentation thresholds, close holes (iterations), and distance-to-nearest neighbor are particularly important for the instrument's ability to detect and measure particles accurately.
Typically, users perform context settings optimization subjectively. Using File Processing Mode, a single sample can be processed under different context settings to determine which settings qualitatively yield the most accurate particle detection. Many users, however, are interested in developing more objective methods for context settings optimization.
 Researchers at U-medico and Osaka University recently published a method for rational optimization of FlowCam settings for analyzing aggregates of proteins and adeno-associated viruses (AAVs). Kurinomaru et al.1 describes using poly(methyl methacrylate) (PMMA) spheres for setting optimization rather than their own samples or a more typical reference standard like ethylene tetrafluoroethylene (ETFE).
Researchers at U-medico and Osaka University recently published a method for rational optimization of FlowCam settings for analyzing aggregates of proteins and adeno-associated viruses (AAVs). Kurinomaru et al.1 describes using poly(methyl methacrylate) (PMMA) spheres for setting optimization rather than their own samples or a more typical reference standard like ethylene tetrafluoroethylene (ETFE).
PMMA spheres have similar optical properties to common biopharmaceutical particles and thus similar context settings can be used. Like polystyrene beads commonly used to validate particle sizing accuracy, but unlike many other types of samples, PMMA spheres can be obtained in a uniform, consistent size. This allows users to optimize their context settings not just on the qualitative accuracy of the segmentation, but also on the quantitative accuracy of the measured size.
The researchers applied this methodology to two FlowCam 8100 units: one with a black and white camera and another with a color camera. While the instrument settings required to achieve optimal sizing on PMMA spheres varied, once optimized, the instruments reported similar measurement values when the same settings were applied to aggregated protein and AAV samples. This demonstrates that the optimized PMMA settings not only are effective at analyzing biopharmaceutical particles but can also be used to harmonize measurements between FlowCam units.
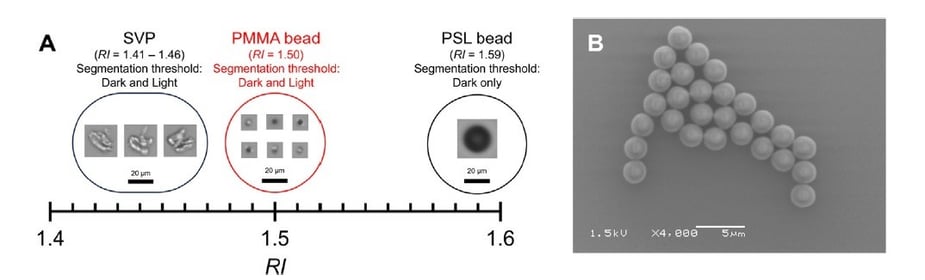
Pictured above: Kurinomaru et al. Figure 11. (A) Comparison of the refractive index (RI) values of subvisible particles (SVPs), polystyrene latex (PSL) beads, and poly(methyl methacrylate) (PMMA) beads. Representative images were obtained by FIM. (B) Scanning electron microscope image of PMMA beads. Particles are uniform in size and highly spherical.
Using PMMA as a surrogate for FlowCam context settings optimization is a worthwhile approach for researchers working with biopharmaceutical samples who would like a more objective method. The PMMA approach can also be an effective method for qualifying the performance of your current settings using a sizing standard that more closely resembles the optical properties of common particle types than typical polystyrene standards. Additionally, this approach may be helpful in optimizing settings for other types of translucent particles, including cells and lipid nanoparticle aggregates.
For researchers interested in exploring the use of PMMA beads with FlowCam 8100 instruments, we recommend using spheres at least 5 μm in diameter. Using smaller particles may make it challenging to validate the optimized settings qualitatively as the particles may only be a few pixels wide. The smaller the spheres, the more important it is to clean the instrument before the analysis — as small beads can be challenging to differentiate from background particles when assessing measurement accuracy. Using 5-10 μm PMMA spheres may make setting optimization easier to perform and help users get the most value out of this promising method.
References
- Takaaki Kurinomaru et al. Optimization of Flow Imaging Microscopy Setting Using Spherical Beads with Optical Properties Similar to Those of Biopharmaceuticals. J Pharm Sci. 2023;112(10). doi:10.1016/j.xphs.2023.10.007