![]() Omontys® – a brand name peginesatide injectable – was voluntarily withdrawn from the market less than a year after the product launch. Clinical trials had demonstrated the drug to be safe and efficacious, but over 40 cases of anaphylaxis, and 7 fatalities were reported soon after the product was introduced to the market.
Omontys® – a brand name peginesatide injectable – was voluntarily withdrawn from the market less than a year after the product launch. Clinical trials had demonstrated the drug to be safe and efficacious, but over 40 cases of anaphylaxis, and 7 fatalities were reported soon after the product was introduced to the market.
Peginesatide, a man-made form of erythropoietin, is used to treat anemia in people with chronic kidney disease treated with dialysis. Those suffering from kidney failure may have reduced amounts of erythropoietin in their body.
A recent article in the Journal of Pharmaceutical Sciences (JPS)1 discussed how the U.S. Food and Drug Administration (FDA) task force investigated the Omontys events with several particle analyzers, including FlowCam.
Omontys was manufactured and approved as both a single-use vial (SUV) and a multiuse vial (MUV), which differed in their formulation. Clinical trials primarily used the SUV formulation, but only the MUV formulation was marketed.
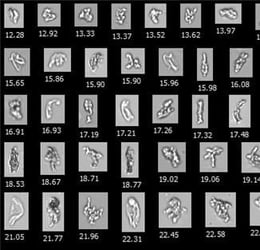
The FlowCam was used to evaluate the particle profile of SUV and MUV samples. The particle images captured by the FlowCam were then analyzed using VisualSpreadsheet®.
The study revealed a significantly higher concentration of subvisible particles in the MUV presentation and correlates linked to the cases of anaphylaxis. Although it is unknown whether the elevated particulate content is related to these serious adverse events of the drug, the report illustrates the importance of capturing and characterizing subvisible particulates.
The peginesatide samples passed USP <788> limits for particulates, but the methods of particle analysis used by the FDA task force suggest the FlowCam can differentiate protein aggregates, silicone oil, and air bubbles from other particles that would otherwise go undetected using light obscuration alone.
Learn more about FlowCam LO. With a single sample and single run, you can obtain LO data for USP compliance, and digital images with flow imaging microscopy for verification.
REFERENCES
1. Subvisible Particle Content, Formulation,and Dose of an Erythropoietin Peptide Mimetic Product Are Associated With Severe Adverse Postmarketing Events
Kotarek, Joseph et al. Journal of Pharmaceutical Sciences 2016, Volume 0, Issue 0, DOI:10.1016/S0022-3549
http://jpharmsci.org/article/S0022-3549(15)00180-X/abstract