Pollen-based microcapsules such as hollow sporopollenin exine capsules (SECs) have emerged as excellent drug delivery and microencapsulation vehicles due to their eco-friendly nature, uniform micron-scale size, and chemical and physical stability. Natural pollen species such as dandelion pollen grains offer diverse architectural features such as large internal cavities and a tough outer exine layer that can be readily prepared and utilized as microencapsulation materials.
The suitability of these pollen microcapsules as drug delivery vehicles has been tested in two recent studies by Fan et al and published in Scientific Reports:
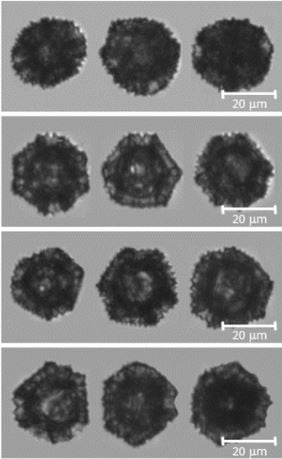
Extraction of cage-like sporopollenin exine capsules from dandelion pollen grains
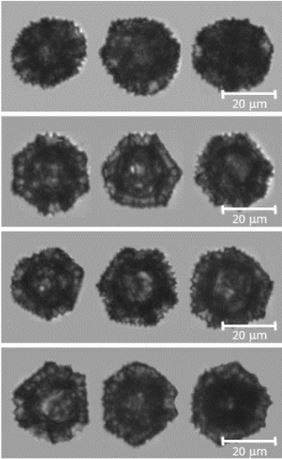
In this study, FlowCam was used, along with other imaging technologies, to assess the preservation of the dandelion SEC morphology during the extraction of inherent proteinaceous materials. The size and morphology was characterized by their distribution of diameter, circularity and aspect ratio.
The study concludes that dandelion SECs could be a promising shell material for microencapsulation and drug delivery.
Pictured at right: dandelion sporopollenin exine capsules as imaged by FlowCam.
Human blood plasma catalyses the degradation of Lycopodium plant sporoderm microcapsules
In this study, sporoderm microcapsules (SDMC) of the Lycopodium and extracted sporopollenin exine capsules (SECs) were identified as being able to withstand the harsh environment of the stomach, which can facilitate the oral administration and controlled drug release into the gastrointestinal (GI) tract. Furthermore, it was reported that when SECs permeate through the intestinal walls and enter the bloodstream, they decompose within 30-60 minutes.
FlowCam was used to monitor particle count and size of SDMCs. Additionally, the morphological changes of the Lycopodium were tracked over time following blood plasma incubation. Flow Imaging Microscopy analysis demonstrates definitive morphological changes following incubation in human blood plasma. This supports the research that human blood plasma catalyses the degradation of Lycopodium plant sporoderm microcapsules.