Plant sporoderm are among the most robust biomaterials in nature. The spore/pollen cell material can be easily extracted leaving an excellent natural microcapsule that can serve as a potential drug delivery mechanism for different biopharmaceutical applications. See related post on dandelion pollen in a similar application.
Plant sporoderm are among the most robust biomaterials in nature. The spore/pollen cell material can be easily extracted leaving an excellent natural microcapsule that can serve as a potential drug delivery mechanism for different biopharmaceutical applications. See related post on dandelion pollen in a similar application.
In this recent paper, sporoderm microcapsules (SDMC) of the Lycopodium and extracted sporopollenin exine capsules (SECs) were identified as being able to withstand the harsh environment of the stomach, which can facilitate the oral administration and controlled drug release into the gastrointestinal (GI) tract. Furthermore it was reported that when SECs permeate through the intestinal walls and enter the bloodstream, they would decompose within 30-60 minutes.
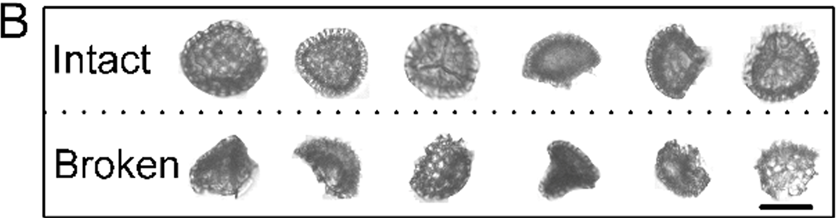
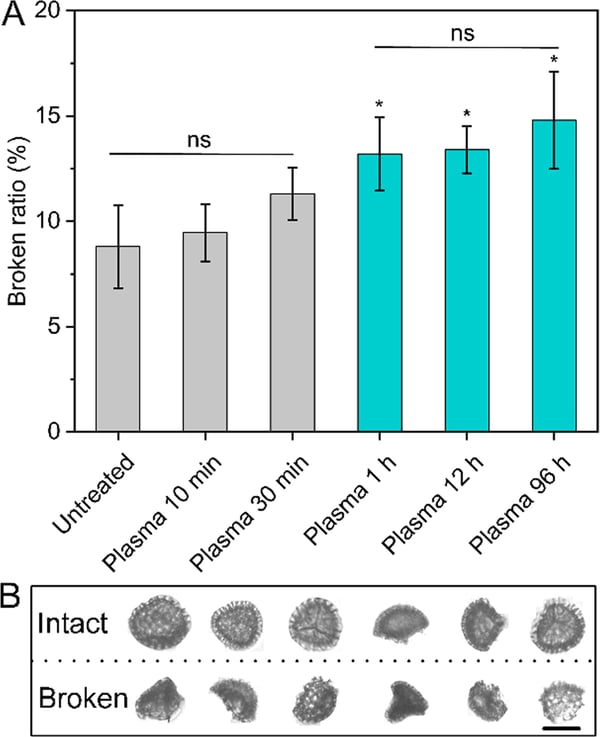 Integrity analysis of Lycopodium SDMCs before and after human blood plasma treatment as measured by FlowCam digital imaging particle analysis. (A) Broken ratio of Lycopodium SDMCs before and after blood plasma treatment; (B) representative FlowCam images of intact and broken spores.
Integrity analysis of Lycopodium SDMCs before and after human blood plasma treatment as measured by FlowCam digital imaging particle analysis. (A) Broken ratio of Lycopodium SDMCs before and after blood plasma treatment; (B) representative FlowCam images of intact and broken spores.
The FlowCam was used to monitor particle count and size of SDMCs. Additionally, the morphological changes of the Lycopodium were tracked over time following blood plasma incubation. Flow Imaging Microscopy analysis demonstrates definitive morphological changes following incubation in human blood plasma. This supports the research that human blood plasma catalyses the degradation of Lycopodium plant sporoderm microcapsules.