Nick Ray was awarded the FlowCam Aquatic Research Student Grant in 2016 as a member of the Fulweiler Lab at Boston University. As a PhD candidate at the time, Nick used FlowCam to investigate phytoplankton community structure at oyster habitats in Narragansett Bay, Rhode Island, and to test how oysters may exert bottom-up control on phytoplankton communities by changing nutrient availability.
“Using FlowCam for a whole summer allowed me to generate a lot of data that contributed to my dissertation work and also helped an undergraduate student complete a Senior Thesis at Boston University. As part of the grant, I attended the Coastal Estuarine Research Federation conference in 2017, where I presented this work and met other scientists in my field. FlowCam was easy to use, and everyone I interacted with on the FlowCam team was incredibly friendly and helpful!” Nick and his colleagues aim to submit one of the manuscripts from their FlowCam research for publication.
Today, Nick is a coastal ecologist and biogeochemist. After completing his Ph.D. at Boston University, he is now an assistant professor at the University of Delaware School of Marine Science and Policy.
Pictured above: Nicholas Ray, during his PhD studies at Boston University, collecting samples in Narragansett Bay, Rhode Island, for his study on the oyster's effect on silica cycling and estuarine health. (Credit: Nicholas Ray)
Over the years since Nick received the FlowCam Equipment Grant, we have had the opportunity to get to know him and reconnected to discuss his research.
Could you walk us through your research? What interests you about oysters' effects on the silica cycle, and why is this important?
Oysters can be described as ecosystem engineers. They alter their environment by filtering the water column and metabolizing food. Because of their amazing filtering capacity (around 170 L per day!), oysters have the power to transform and recycle carbon and nutrients, profoundly impacting water quality and sediment characteristics.
My dissertation focused on how oysters and different oyster habitats change biogeochemical cycling in a temperate estuary (Narragansett Bay, Rhode Island). Oyster populations are increasing along the East Coast of the U.S. primarily because of growing oyster aquaculture and, to a lesser extent, restoration efforts.
Silica is one of the nutrients I studied. It is an important regulator of diatom productivity and abundance. When water column concentrations of dissolved silica are greater than dissolved nitrogen, diatoms tend to dominate the phytoplankton community. Diatoms form the base of many coastal food webs and transfer more energy between trophic levels than other phytoplankton groups, such as dinoflagellates or cyanobacteria. Dinoflagellates and cyanobacteria are also associated with poor water quality and harmful algal blooms. Therefore, higher dissolved silica concentrations can be associated with improved water quality.
Some evidence shows that oysters may change estuarine silica cycling by stimulating sediment microbial processes. Many microorganisms that live in estuarine sediment create energy by decomposing the particulate matter that sinks into the sediment. The oyster filter-feeding process moves particles suspended in the water column to the sediment as oysters feed and produce feces and when oysters reject unfavorable particles as pseudo-feces. The feces and pseudo-feces sink to the sediment much more rapidly than the suspended particulates the oyster feeds on, increasing the amount of organic material available for microbial decomposition in the sediment near oysters. The more organic matter available for sediment microbes, the higher the respiration rates and recycling of nutrients, like silica. These nutrients are then released from the sediment into the water column. Through this process, oysters promote a greater concentration, or availability, of silica for diatoms.
 Nicholas Ray in the laboratory at Boston University. Ray used the FlowCam to analyze phytoplankton communities present in sediment core samples. (Credit: Nicholas Ray)
Nicholas Ray in the laboratory at Boston University. Ray used the FlowCam to analyze phytoplankton communities present in sediment core samples. (Credit: Nicholas Ray)
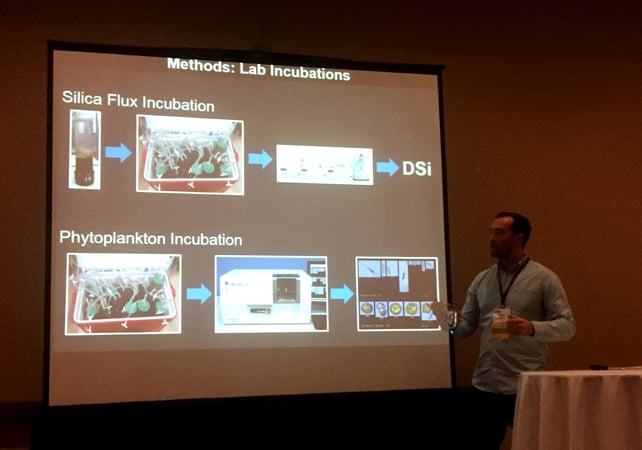
Using a two-part experiment, I tested whether oyster-mediated sediment silica regeneration can change the phytoplankton community. I first collected sediment cores from oyster and non-oyster habitats in Narragansett Bay. I brought them to the lab, where I used an "incubation" technique to measure nutrient exchange rates between the sediment and water column. The technique involves keeping the cores in a water bath of constant temperature, then capping the core and taking small samples of water over time as the dissolved oxygen concentration in the water in the core decreases. The decrease in dissolved oxygen during this incubation period informs me that microbial activity is occurring in the sediment. After incubation, the collected water samples are analyzed to determine their silica concentration. I can then calculate a silica “flux” as the change in silica concentration over time. A positive flux indicates the release of silica from the sediment, and a negative flux tells me that the sediment is actively taking silica from the water column. Following the silica incubation, I removed the caps from the cores and replaced the water with water collected from my field sites. I analyzed the phytoplankton community in the water column of each core (with FlowCam) over a four-day period to look for changes in the relative abundance of diatoms and dinoflagellates related to the measured silica flux.
The results suggest greater rates of silica regeneration from sediment collected in oyster habitats compared to non-oyster habitats. There appears to be an increase in dinoflagellate populations in water incubated over sediment collected from non-oyster habitats. The phytoplankton community composition in water incubated over sediment from oyster habitats did not change. These results suggest that oysters may not promote increased diatom abundance but may serve more as a “phytoplankton community manager,” maintaining the present community and not allowing dinoflagellates to take over.
How did you use FlowCam?
I used FlowCam to analyze the phytoplankton species present in the sediment cores I collected from different oyster habitats. I sampled the water from a total of 30 sediment cores over a 4-day period using trigger mode on the FlowCam VS at 4x and 20x magnification. I then analyzed the data collected by FlowCam using the Visual Spreadsheet software and assigned individual particles that were identified to functional groups (e.g., diatoms and dinoflagellates). The high sample throughput rates of the FlowCam allowed me to generate a large amount of data in a short time from multiple experimental trials and field sites.
What was your grant experience like? How could it have been better?
Having access to a FlowCam instrument for the summer allowed me to add a chapter to my dissertation research. Without FlowCam, I would not have been able to complete this project. The initial instrument, software training, and all subsequent interactions with the FlowCam team were helpful and enjoyable.
Traveling to the CERT conference in Providence to present my work was a highlight and an exciting opportunity. I met and talked with other scientists who research the relationship between oysters and estuarine biogeochemistry.
 Nicholas Ray presented his research at CERF 2017. (Credit: Nicholas Ray)
Nicholas Ray presented his research at CERF 2017. (Credit: Nicholas Ray)
What inspired you to pursue a career in marine research?
Understanding the local and global impacts of the food we eat is important. My dissertation research focused on oyster aquaculture. I like researching oysters because they change biogeochemical processes in ways that could benefit ecosystems. Estuaries and oceans have untapped potential for sustainable food production, and I also enjoy conducting fieldwork in and around the water!
Thanks, Nick, for taking the time to discuss your research with us. We look forward to following your research and wish you continued success!
Interested in learning more about how FlowCam is used for aquaculture?
Download the App Note for Shellfish Aquaculture
You can read the abstract from Nick's thesis, entitled "Oyster Regulation of Biogeochemical Cycling in Temperate Estuaries," below.
"Of the many changes humans have caused in coastal systems, excess nutrient loading is perhaps the most dramatic. Specifically, excess nitrogen (N) can lead to a series of negative consequences, such as eutrophication, low oxygen conditions, and decreased biodiversity. Concurrent with changes in nutrient loading, coastal shellfish populations have been devastated by over-harvesting, disease, and pollution. For example, oyster reefs – once a dominant feature along many coastlines – have been reduced by 85% of their historic range globally. Today, oysters are returning to coastal systems through restoration projects and a boom in aquaculture. Yet the impact of returning oysters to coastal systems is unknown. My dissertation helps to fill this major knowledge gap. Specifically, this dissertation focuses on oysters' role in regulating coastal nutrient cycling and greenhouse gas (GHG) emissions.
In chapter one, I estimated the GHG cost of protein production using oyster aquaculture. Using a combined field and laboratory approach, I quantified rates of N2O, CH4, and CO2 release from cultured oysters, and changes in sediment fluxes of these GHGs. On a kg CO2-equivalent kg-1 protein produced, oyster aquaculture has less than 0.5% of the GHG cost of terrestrial livestock production. In chapter two, I used an oyster aquaculture chronosequence to examine how organic matter loading from oysters drove sediment N cycling processes to an alternative stable biogeochemical state. I found that sediments under oyster aquaculture oscillated over time, shifting between N removal (N2) and recycling (NH4+) processes, demonstrating non-linear dynamics. In chapter three, I demonstrate that sediment N cycling processes in oyster habitats follow seasonal patterns of water column productivity, recording net denitrification in the spring following a phytoplankton bloom and net nitrogen fixation in the fall. In chapter four, I use a meta-analysis approach to describe past and future oyster population changes in the context of “biogeochemical sustainability.” I show that oysters stimulate both N removal and recycling across systems at a minimal GHG cost and conclude that larger oyster populations will yield more biogeochemically sustainable coastal systems."











