All types of biotherapeutics, ranging from protein therapies to cell and gene therapies, contain particles. While the types of particles can vary between therapies, these particles can indicate important changes in how a sample was prepared or handled and can pose issues during quality control and in the clinic. Particle monitoring strategies are critical in biotherapeutic development to assess product quality, meet regulatory guidelines like USP <788>, and ensure product safety and efficacy.
Flow imaging microscopy (FIM) is increasingly used to measure the concentration, size, and morphology of subvisible (2-100 µm) particles in their native buffer. The technique uses high-throughput, high-quality microscopy imaging of particles to help researchers identify and control the sources of unwanted particles and improve product quality. It is recommended as an orthogonal method to light obscuration for quality control applications via USP <1788>. FIM can also work alongside other complementary and orthogonal particle analysis techniques as described in a recent webinar.
FlowCam is a flow imaging microscope used in the development of many biotherapeutic platforms ranging from traditional protein therapies to cell therapies, gene therapies, and other advanced biologics. Below, we provide an overview of how FlowCam data can be used to support the development of common biotherapeutics alongside helpful resources covering applications for each therapy type in more detail.
Protein Therapies:
 Protein aggregates are ubiquitous in protein-based drug products and have been associated with anti-drug antibody formation and other adverse reactions in the clinic. FlowCam allows researchers to differentiate these aggregates from other common inherent, intrinsic, and extrinsic subvisible particles like silicone oil, degraded polysorbate, and glass flakes from each other. FlowCam can also be used to distinguish between protein aggregates generated by different sources, helping users identify and mitigate the stresses these samples are exposed to during manufacturing, shipping, storage, and use.
Protein aggregates are ubiquitous in protein-based drug products and have been associated with anti-drug antibody formation and other adverse reactions in the clinic. FlowCam allows researchers to differentiate these aggregates from other common inherent, intrinsic, and extrinsic subvisible particles like silicone oil, degraded polysorbate, and glass flakes from each other. FlowCam can also be used to distinguish between protein aggregates generated by different sources, helping users identify and mitigate the stresses these samples are exposed to during manufacturing, shipping, storage, and use.
The white paper, Characterizing Protein Aggregation With Orthogonal and Complementary Analytical Techniques, describes how flow imaging microscopy using FlowCam LO and FlowCam Nano is combined with other analytical techniques, such as light obscuration and dynamic light scattering to characterize protein aggregation in protein formulations.
Another white paper, Robust AI Methods for Protein Biotherapeutics, demonstrates how FlowCam images can discriminate between particle types in protein formulations and, specifically, artificial intelligence software such as VisualAI to differentiate between subvisible protein aggregates and silicone oil droplets. Other types of intrinsic and extrinsic particles and different types of protein aggregates can also be identified in this fashion.
Cell Therapies:
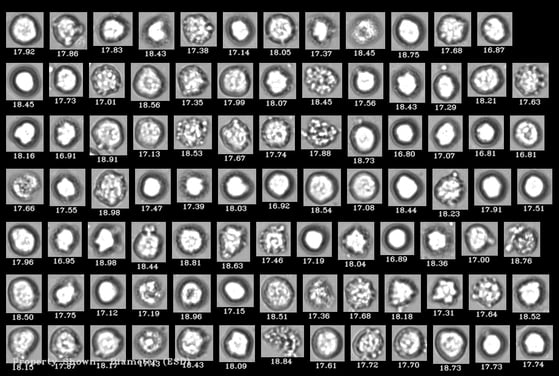 FlowCam 8000 series instruments are powerful cell analysis platforms, combining microscopy's imaging and cell analysis capabilities with the throughput of flow cytometry. The instrument provides quantification of cells and other prevalent contaminants in cell therapies, including cell aggregates, cellular debris, and residual Dynabeads™. Flow imaging microscopy can also be used as a high-throughput, label-free measurement of cell viability, using cell morphology rather than fluorescent labeling to identify viable cells. This information can help identify hard-to-remove particulate contaminants in these therapies and eliminate them at the source.
FlowCam 8000 series instruments are powerful cell analysis platforms, combining microscopy's imaging and cell analysis capabilities with the throughput of flow cytometry. The instrument provides quantification of cells and other prevalent contaminants in cell therapies, including cell aggregates, cellular debris, and residual Dynabeads™. Flow imaging microscopy can also be used as a high-throughput, label-free measurement of cell viability, using cell morphology rather than fluorescent labeling to identify viable cells. This information can help identify hard-to-remove particulate contaminants in these therapies and eliminate them at the source.
As an example application, a recent study by Grabarek et al. demonstrates how FlowCam images in combination with artificial intelligence-driven image analysis tools can be used to differentiate between viable and nonviable cells as well as cellular debris. This approach allowed the researchers to obtain rapid cell viability measurements for a simulated cell therapy upon exposure to stresses encountered in manufacturing.
Gene Therapies:
 Like proteins, common viral vectors used in cell and gene therapy like AAVs, adenoviruses, and lentiviruses will aggregate over time, resulting in particles that pose product quality concerns. Traditional particle analysis techniques often require prohibitively large sample volumes for many gene therapy products—especially early in development. FlowCam offers flexible sample volume requirements, providing particle detection and source information with as little as 100 µL as well as robust particle quantification with larger aliquots later in development and production. No other protocol changes are required to analyze a different sample volume. This flexibility allows researchers to obtain appropriate viral vector aggregate and particle characterization at any stage of gene therapy development with as little or as much volume as is available and deemed appropriate for analysis.
Like proteins, common viral vectors used in cell and gene therapy like AAVs, adenoviruses, and lentiviruses will aggregate over time, resulting in particles that pose product quality concerns. Traditional particle analysis techniques often require prohibitively large sample volumes for many gene therapy products—especially early in development. FlowCam offers flexible sample volume requirements, providing particle detection and source information with as little as 100 µL as well as robust particle quantification with larger aliquots later in development and production. No other protocol changes are required to analyze a different sample volume. This flexibility allows researchers to obtain appropriate viral vector aggregate and particle characterization at any stage of gene therapy development with as little or as much volume as is available and deemed appropriate for analysis.
Particulates in gene therapies are of growing concern to researchers who want to ensure the safety of these advanced biotherapeutics, especially as common particle removal strategies like filtration can adversely impact efficacy. A recent publication by Molina et al. calls on researchers and regulatory agencies to develop standards and best practices for monitoring particles in cell and gene therapies to improve their safety—efforts that FlowCam instruments can support.
Nanomedicines:
 Lipid nanoparticles (LNPs), liposomes, exosomes, and other drug delivery vehicles are of interest to researchers due in part to the success of the COVID-19 vaccines. FlowCam can be used to monitor the stability of nanomedicines in a formulation and can detect degradants such as aggregates. With FlowCam Nano, researchers can potentially detect the oligomers these drug delivery vehicles can form and detect changes in product quality as soon as they occur.
Lipid nanoparticles (LNPs), liposomes, exosomes, and other drug delivery vehicles are of interest to researchers due in part to the success of the COVID-19 vaccines. FlowCam can be used to monitor the stability of nanomedicines in a formulation and can detect degradants such as aggregates. With FlowCam Nano, researchers can potentially detect the oligomers these drug delivery vehicles can form and detect changes in product quality as soon as they occur.
As an example, our application note, Flow Imaging Microscopy to Monitor Lipid Nanoparticle Aggregation, discusses how FlowCam 8000 series instruments and FlowCam Nano can be used to monitor the particle content present in LNP-based drug products, including small LNP oligomers.
Regardless of the type of biotherapeutic you work with, FlowCam instruments can help detect, quantify, and analyze relevant particle types in your therapies. This information can help you identify and mitigate unwanted particles, ensure regulatory compliance, and maximize the efficacy of your therapies in the clinic.











