While analysis with light obscuration is standard, the FDA has long made clear that size data alone, collected with light obscuration, is not adequate to ensure safe and effective drugs. Orthogonal methods such as flow imaging microscopy are necessary to provide validation.
With the FlowCam LO, the first and only light obscuration (LO) instrument with imaging capabilities is now available. This easy-to-use instrument delivers a powerful, all-in-one solution to meet compliance standards while allowing verification with high-resolution images.
Explore our new technology in the video below.
Does your lab rely upon Light Obscuration (LO) for USP standards for particle size measurement?
While LO determines size distributions, it provides no details about the shape and nature of the particles in your sample.
Two scenarios where light obscuration alone is insufficient:
- Light obscuration may not characterize the size of protein aggregates accurately, given that their refractive index is close to that of the buffer solution.
- Light obscuration quantifies particles in terms of an equivalent spherical diameter (ESD). Therefore, particles of different origins (intrinsic, extrinsic, inherent) can not be distinguished.
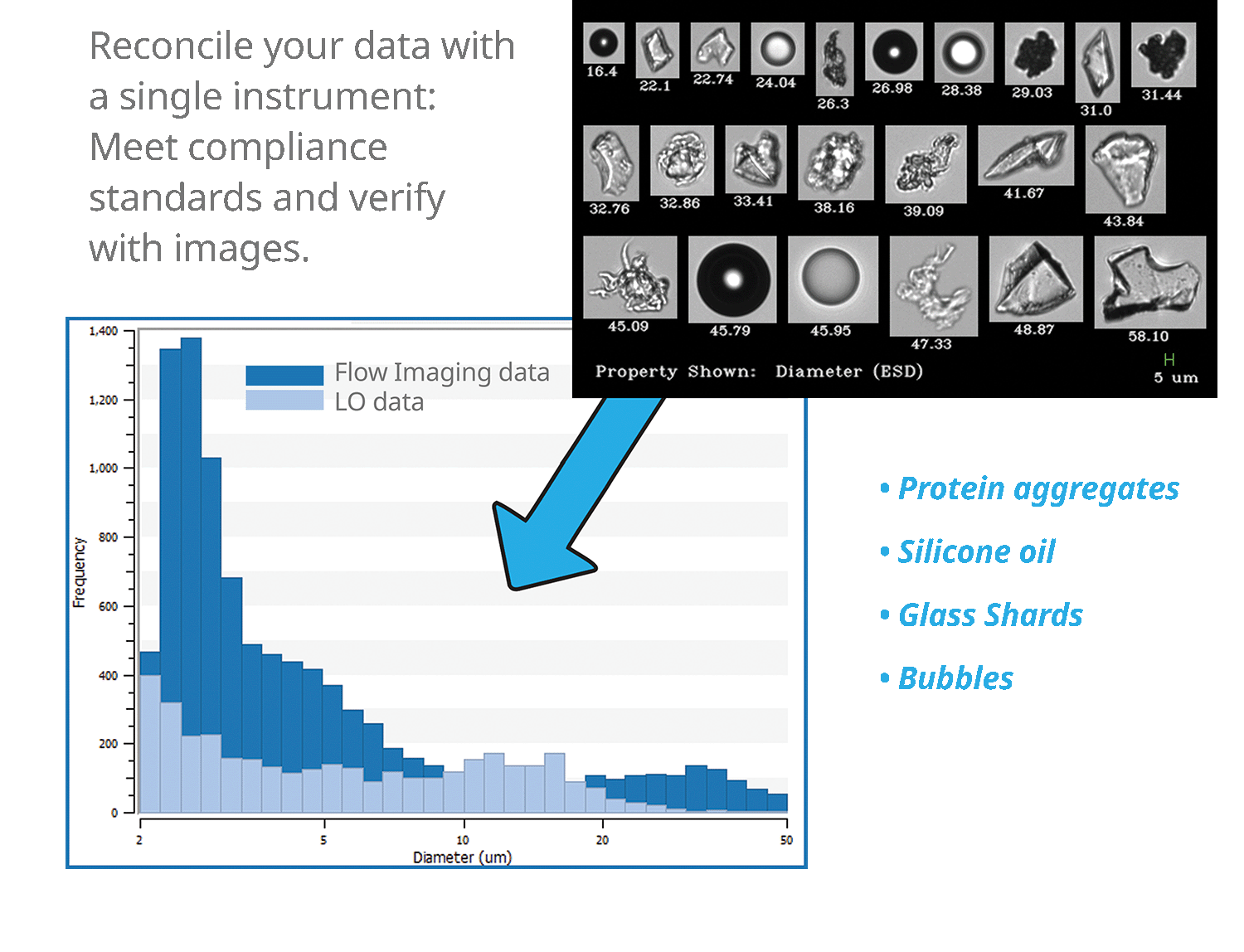
The image below shows the variety of particles that might be obtained from flow imaging that have a similar size. Shown here: proteins, agglomerates, silicone droplets, air bubbles and other contaminants - silica, glass shards.
.jpg?width=577&name=FlowCam%208000%20Biopharm%20Collage%206.2.20%20(2).jpg)
Want to learn more about the technology?
Explore our new white paper: Measuring Subvisible Particles and Aggregates Using FlowCam LO