West Pharmaceutical Laboratories (Exton, PA) recently published a paper on a streamlined bioanalytical approach to determine the most appropriate primary container system early in drug development. Given the importance of monitoring biological drug and container interactions to ensure drug and patient safety, it is necessary to consider multiple variables when determining drug packaging systems.
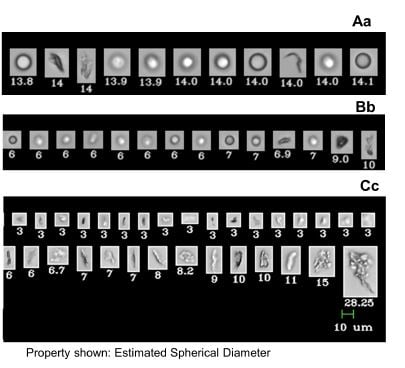
In this paper they tested the stability of a commercially available mAb in three syringe types under three conditions: cold storage, high temperature, and agitation induced stress. They used the FlowCam to differentiate mAb stability as measured by aggregate formation in different syringe systems. It was shown that Flow Imaging microscopy (FlowCam) is a sensitive analytic technique that can help predict which primary container system will provide higher stability when the drug is stored under standard conditions for period of time longer than 60 days.
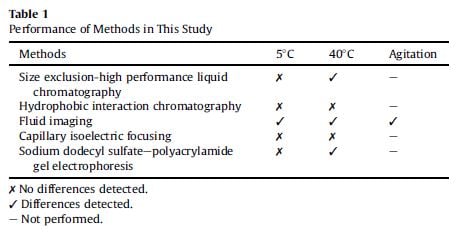
Different methods were used to compare the stability of the formulation at various stages of the testing. They compared the sensitivity of these different methods to detection of aggregates in each of the syringes. The table below compares the results of different systems and their ability to detect differences in the formulation.
Ranjana Singh, PhD *, Lloyd Waxman, PhD
Scientific Insights Lab, West Pharmaceutical Services, Inc., Exton, Pennsylvania 19341
Read the paper here at the Journal of Pharmaceutical Sciences.