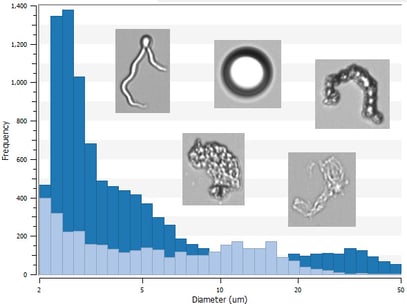
Effective strategies for monitoring particles in biotherapeutics are critical to meet regulatory requirements like USP <788> and mitigate the potential safety risks that particles pose. Flow imaging microscopy (FIM) is a common particle characterization approach used to measure the number, size distribution, and types of subvisible particles (2-100 µm in size) in biotherapeutic samples. This information can help assess product stability and quality.
This webinar discusses how FIM instruments can help characterize particle impurities in different biotherapeutic platforms when paired with orthogonal and complementary analytical techniques. The session primarily focuses on a case study in which FIM and complementary particle analysis techniques including light obscuration (LO) and dynamic light scattering (DLS) were used to analyze particles in protein formulations. These measurements were used to determine the impact of stress conditions and surfactants on protein aggregation during an accelerated stability study. Strategies for monitoring cells, viral vector aggregates, and other particles encountered in cell and gene therapy samples are also discussed.
Learning Objectives:
- The particle properties flow imaging microscopy provides and how they relate to other particle analyses methods
- The information orthogonal and complementary particle analysis techniques provide researchers in biotherapeutic development
- The utility of particle measurements in developing protein and cell & gene therapies
 Learn more about FlowCam
Learn more about FlowCam
FlowCam offers a full portfolio of particle imaging instruments to count, size, and determine the morphology of particles from 300 nm to more than 100 µm in size in biopharmaceutical formulations, including protein, cell, and gene therapies.
FlowCam for Protein and Cell & Gene Therapy Applications