 Savannah Mapes (pictured here) was a graduate student in the Reece Lab at the Virginia Institute of Marine Science (VIMS) when she was awarded the use of a FlowCam 8000 to study harmful algal blooms (HABs) in the York River/lower Chesapeake Bay area.
Savannah Mapes (pictured here) was a graduate student in the Reece Lab at the Virginia Institute of Marine Science (VIMS) when she was awarded the use of a FlowCam 8000 to study harmful algal blooms (HABs) in the York River/lower Chesapeake Bay area.
Marine HABs are most frequently caused by a diverse group of phytoplankton called dinoflagellates. HABs can be dangerous due to their ability to produce toxins and/or deplete dissolved oxygen used by fish and other organisms. Mapes set out to better understand two dinoflagellates in particular: Alexandrium monilatum and Margalefidinium polykrikoides (affectionately known as “Alex” and “Marg,” respectively).
Mapes conducted extensive fieldwork during the late summer to collect samples from blooms as they occurred. "I went out into the York River every day possible and collected samples from every visible algal bloom, which provided me with FlowCam images and cell counts," wrote Mapes.
Keep reading to learn more about her research and how FlowCam helped with cell counting and enabled her to collect the necessary data for a poster presentation at the US Symposium on Harmful Algae.
 Mapes is pictured here on one of her many sampling trips in the Chesapeake Bay area. Samples were taken back to VIMS for FlowCam processing.
Mapes is pictured here on one of her many sampling trips in the Chesapeake Bay area. Samples were taken back to VIMS for FlowCam processing.
Mapes observed M. polykrikoides blooms around the area late last summer, including one in the York River, followed by an A. monilatum bloom. “This has typically been the pattern in the York River since 2007,” Mapes observed, but a shift in weather conditions in 2018 that included increased rainfall and cooler summer temperatures disrupted that pattern – until now.
“For the first time in 3 years, we had the opportunity to study and document harmful algae blooms during the late summer in the York River,” Mapes wrote in her grant proposal.
HABs can visibly change the color of the water, as shown here during an Alexandrium bloom (left). A bloom can sometimes be so dense that individual chains are visible to the naked eye (right).

|

|
VIMS normally documents daily species concentrations and cell counts using light microscopy and quantitative PCR (qPCR), which they continued to do through the grant period. One of the benefits of the FlowCam for supplementing this type of work, Mapes noted, was the amount of data that can be collected from a single milliliter of water, including a “visual representation of the microscopic community, classification, and enumeration of cells, and the dimensions of every particle captured.”
“Alexandrium monilatum is a tricky species to cell count due to its long-chain-forming behavior,” Mapes wrote. “With FlowCam, I could filter different sized chains into corresponding categories with a defined number of cells, which was an efficient method for doing cell counts.”
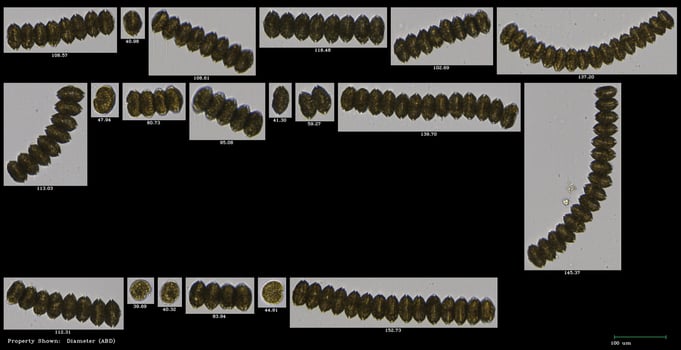
Pictured here are examples of one of Mapes’ A. monilatum classifications in VisualSpreadsheet. A. monilatum is a chain-forming species of dinoflagellate, making counting individual cells very time-consuming. By grouping chains of the same length together, Mapes found that FlowCam was an efficient way to achieve the cell counts she needed.
In addition to monitoring natural blooms, Mapes also conducted lab-based experiments using A. monilatum cultures to better understand their complex life cycle, which includes both sexual and asexual reproduction. This work will form the foundation of her Master’s thesis.
“While in nature we have often observed sexual reproduction as A. monilatum blooms progress, we have not been able to induce sexual reproduction in the lab to date,” wrote Mapes’ advisor, Dr. Kim Reece, in her proposal. “[The] FlowCam will be used to examine the cultures following the different treatments and determine whether they are going through the same life stages that we observe in the field samples based on the classifications that were done with York River samples.”
In the lab, Mapes experimented with various culture conditions to try to induce sexual reproduction in A. monilatum.
“We have observed many stages of its life cycle,” Mapes reflected at the end of the grant period, “but some need to be examined closer with nucleic acid content studies to determine if they are truly what they seem. There is some curiosity about whether A. monilatum cysts asexually, as is seen in some species. But what we know is that they do form cysts, both pellicle (“temporary) and dormant (“resting”)…We are not sure when meiosis happens, what triggers sexual reproduction, or if there is a mandatory dormancy period for the resting cysts…These are all things I aim to study next!”
While FlowCam was a useful tool for studying naturally occurring blooms and cultures in the lab, this semester was not without many challenges posed by the ongoing pandemic.
“This semester was like sprinting a 100-meter hurdle race; so many hurdles to leap in a very short amount of time,” Mapes said. “COVID-19 hardships, restrictions and regulations, accelerated courses, and political turmoil hampered research capabilities by limiting accessibility to campus, sample collection, available help, and increased mental and physical fatigue.”
Although exhausted, Mapes said, “There was no way I would let this incredible opportunity get away from me.”
“The FlowCam jump-started the first chapter of my graduate thesis, focused on the life cycle of A. monilatum. I now have a visual representation of my species in multiple life phases, information on the community composition of multiple local harmful algal species (in bloom), and numerical data of cell counts for each sample…Considering time requirements and accuracy, I will also compare manual (light microscopy), FlowCam, and qPCR cell count methods.”
Researchers like Mapes are working towards advancing our understanding of the complicated life cycle of HAB species like A. monilatum. The figure below illustrates how tools like FlowCam and microscopes can be used to document the known life stages of these complex organisms. “We have observed sexual reproduction through fusing gametic cells and double-flagellated cells (larger, darker colored cells, the product of the fusing gametes),” wrote Mapes. “It is evident that they also reproduce asexually, as we see with the long chains (a product of binary fission).”
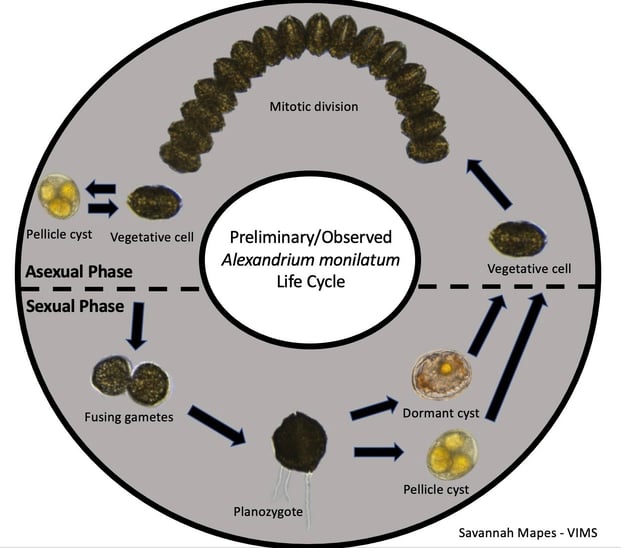 Cyst images were captured by Bill Jones (VIMS) with a light microscope; Mapes captured the remaining images on FlowCam.
Cyst images were captured by Bill Jones (VIMS) with a light microscope; Mapes captured the remaining images on FlowCam.
According to Mapes, FlowCam's single biggest advantage is its “visual representation of the microscopic community, classification, and enumeration of cells, and dimensions of every particle captured.”
This grant provides access to FlowCam and VisualSpreadsheet software, training, and financial support for attending a scientific conference. It is an important opportunity for early-career scientists to present their research and network with other scientists in their field.
Her poster concludes that "The FlowCam is an efficient alternative to manual cell counts, providing more data (particle images and parameters) in less time. The results of all three cell counting methods are comparable for M. polykrikoides. With A. monilatum, the Sedgewick Rafter and FlowCam methods are approximate, but the qPCR method greatly overestimates. It is possible that this is due to using a single clonal culture as the standard for qPCR. rDNA variability among strains is observed for other Alexandrium species. In addition, multiple life stages with different ploidy levels are observed in field samples. Additional clonal cultures are being established to obtain additional qPCR curves.
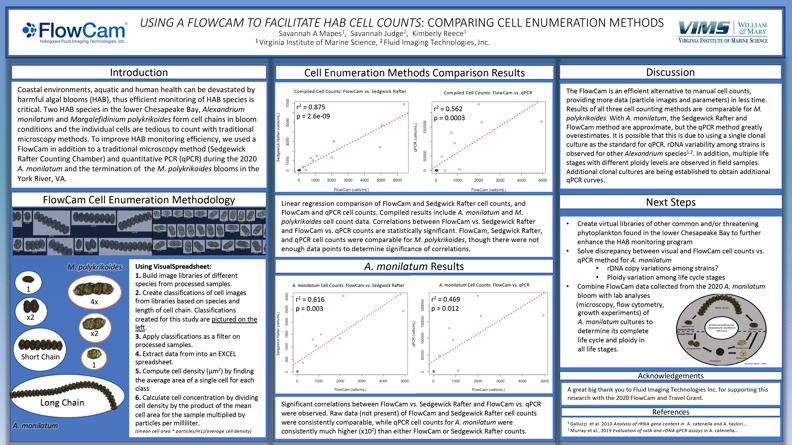
“Any lab with a phytoplankton or harmful algal bloom monitoring program would benefit from having a FlowCam,” Mapes said. “Every HAB lab needs a FlowCam!”











