Subvisible particles in biotherapeutics are essential quality indicators due to their implications for regulatory compliance and potential safety …
Read Post
In a recent paper discussing particle counting and analytical techniques, the number and type of particles present in intravitreal injection …
Read Post
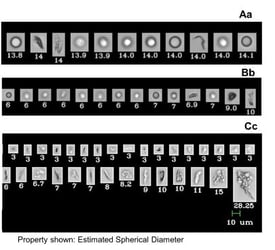
West Pharmaceutical Laboratories (Exton, PA) recently published a paper on a streamlined bioanalytical approach to determine the most appropriate …
Read Post
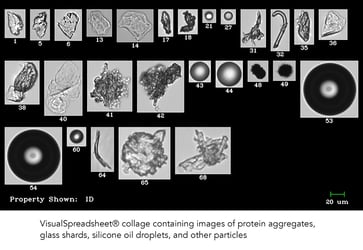
The problem of protein aggregation and the presence of extrinsic particles in biopharmaceutical formulations is not a small one. With the improvement …
Read Post
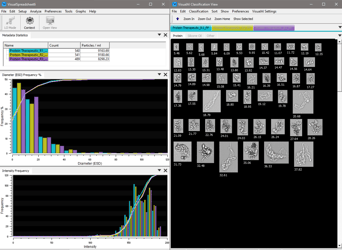
Beyond Particle Counting: Verifying Particle Identity Your current particle analyzer counts the subvisible particles in your protein therapeutic. A …
Read Post
Omontys® – a brand name peginesatide injectable – was voluntarily withdrawn from the market less than a year after the product launch. Clinical …
Read Post

AB BioTechnologies, now Labyrinth Biopharma, is a privately held laboratory offering pharmaceutical contract services in Bloomington, Indiana. …
Read Post






