 Transferring live cells and genetic material directly into a patient is a treatment strategy seeing unprecedented FDA approval rates. There is now a greater need to move the discussion toward particulate management in cell- and gene-specific regulatory guidance1. The particulate matter generated during the CGT manufacturing process needs to be carefully monitored to ensure the continued safety and efficacy of these injectable therapeutics.
Transferring live cells and genetic material directly into a patient is a treatment strategy seeing unprecedented FDA approval rates. There is now a greater need to move the discussion toward particulate management in cell- and gene-specific regulatory guidance1. The particulate matter generated during the CGT manufacturing process needs to be carefully monitored to ensure the continued safety and efficacy of these injectable therapeutics.
CGTs are a diverse class of therapeutic drugs with distinct particulate-related attributes, and, like other parenteral drug products, many types of particles may be found in their preparations that could potentially cause patient harm. In a recent paper published in Cytotherapy1, the authors put forth a call to action to the regulatory guidance and advanced drug development communities to work together to define best practices in particulate prevention and to establish consensus on particulate standards that can be used to assess CGT safety. The challenge is that CGTs (living cells, viral particles, lipid nanoparticles, and exosomes) are particulate in nature, making it difficult to discern inherent drug particles from intrinsically- or extrinsically-derived particle contamination.
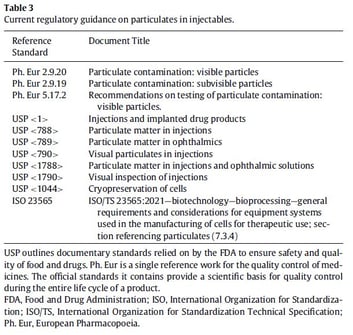 To date, particulate characterization by size alone in CGT-based products has followed regulatory methods established for other biologic and small-molecule drugs – but these methods lack the detection clarity demanded by CGT-specific products. Current compendial regulations for particulates in injectables outlined by the USP and relied on by the FDA include visual inspection and light obscuration techniques (Table 31) that are solely insufficient for CGT drugs. Furthermore, regulatory guidelines for removing large particulate load through filtration and centrifugation are not always suitable for CGTs as they may adversely affect CGT efficacy. As the authors in Molina et al, 2022 point out, there is a clear and present need for product-specific requirements to guide CGT development and manufacturing process optimizations.
To date, particulate characterization by size alone in CGT-based products has followed regulatory methods established for other biologic and small-molecule drugs – but these methods lack the detection clarity demanded by CGT-specific products. Current compendial regulations for particulates in injectables outlined by the USP and relied on by the FDA include visual inspection and light obscuration techniques (Table 31) that are solely insufficient for CGT drugs. Furthermore, regulatory guidelines for removing large particulate load through filtration and centrifugation are not always suitable for CGTs as they may adversely affect CGT efficacy. As the authors in Molina et al, 2022 point out, there is a clear and present need for product-specific requirements to guide CGT development and manufacturing process optimizations.
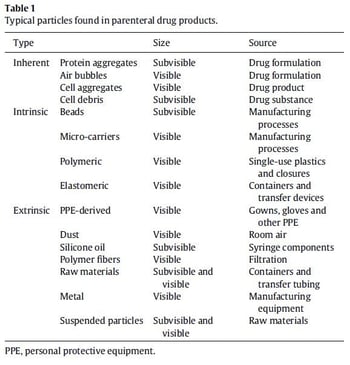 In May 2021, the USP published changes to chapter <1788> that introduced flow imaging as a recommended approach for determining particulate matter in injectable and ophthalmic solutions. FlowCam flow imaging microscopy uniquely fits the revised <1788> recommendations as a complementary, orthogonal method for CGT-based therapeutic characterization. With FlowCam, CGT-product risks can be assessed using imaged-based analyses of visible, subvisible, and submicron particles (Table 11). And with advanced FlowCam VisualSpreadsheet software, discriminatory particle analysis provides real-time feedback on the size, shape, count, and identity of the particulate content in CGT-based parenteral drug products.
In May 2021, the USP published changes to chapter <1788> that introduced flow imaging as a recommended approach for determining particulate matter in injectable and ophthalmic solutions. FlowCam flow imaging microscopy uniquely fits the revised <1788> recommendations as a complementary, orthogonal method for CGT-based therapeutic characterization. With FlowCam, CGT-product risks can be assessed using imaged-based analyses of visible, subvisible, and submicron particles (Table 11). And with advanced FlowCam VisualSpreadsheet software, discriminatory particle analysis provides real-time feedback on the size, shape, count, and identity of the particulate content in CGT-based parenteral drug products.
The Molina manuscript brings the need for regulatory standards to the attention of all CGT stakeholders. The authors provide recommendations to consider while establishing new CGT manufacturing and assessment paradigms including these key questions:
- What type of particulates need to be measured?
- What size and quantity of particulates are acceptable based on dose ranges, route of administration, and therapeutic mechanism of action?
- What are the acceptable final release assays for particulate testing of the final drug product?
- What are the potential implications to patient safety?
- How should we measure different particulates, particularly within cell suspensions?
1. Molina SA, Davies SJ, Sethi D, et al. Particulates are everywhere, but are they harmful in cell and gene therapies? Cytotherapy. 2022 Dec;24(12):1195-1200. doi: 10.1016/j.jcyt.2022.07.014. Epub 2022 Sep 27. PMID: 36175323.