Many new types of biotherapeutic Active Pharmaceutical Ingredients (API) such as viruses, nanomedicines including virus-like particles and lipid nanoparticles, and cell-based medicinal products have recently experienced a significant surge in interest. Like proteins before them, formulations of these APIs contain particles that need to be characterized to ensure product quality and efficacy. Particle characterization technologies like FlowCam thus continue to play a critical role in developing and manufacturing safe biotherapeutics regardless of the API.
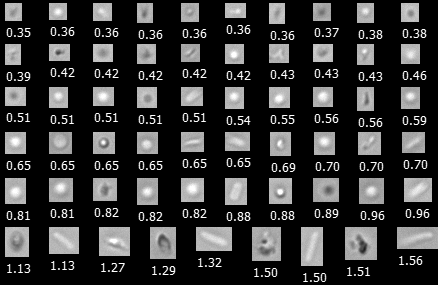
Pictured above, protein aggregates, sucrose particles, and E. coli as imaged by FlowCam Nano. Equivalent spherical diameter (in µm) for each particle is shown below their image.
In a recent paper, Particles in Biopharmaceutical Formulations, Part 2: An Update on Analytical Techniques and Applications for Therapeutic Proteins, Viruses, Vaccines and Cells, the authors review several applications for new and existing particle characterization technologies in developing these new types of biotherapeutics.
The review highlights applications for FlowCam in differentiating between protein aggregates, extrinsic particles, and container-closure associated particles in protein formulations as well as counting and characterizing cells in cell-based medicinal products.
 The article discusses particle characterization techniques for a wide variety of biotherapeutics including proteins, cells, viruses, and nanomedicines like lipid nanoparticles. The authors cover a diverse set of techniques with applications ranging from determining the content of virus particles to analyzing the morphology of API aggregates and other large particles.
The article discusses particle characterization techniques for a wide variety of biotherapeutics including proteins, cells, viruses, and nanomedicines like lipid nanoparticles. The authors cover a diverse set of techniques with applications ranging from determining the content of virus particles to analyzing the morphology of API aggregates and other large particles.
The publication also serves as an update to Particles in Therapeutic Protein Formulations, Part 1: Overview of Analytical Methods which covers Flow Imaging Microscopy (FIM) and other particle characterization technologies in therapeutic protein formulation development. As such, they also discuss several new particle characterization technologies that were released since their initial analysis in 2012.
Included in the discussion of recent innovations in FIM-based particle analysis are FlowCam Nano and machine learning techniques for analyzing FlowCam images. Shortly after the publication of this article, we have introduced the next generation FlowCam Nano for improved ease of use. Read more about the new FlowCam Nano.