In a recent study by Kiyoshi et al., a Japanese consortium conducted a collaborative study to assess the standardization of flow imaging microscopy (FIM) for the analysis of subvisible particles (SVPs) and protein aggregates in therapeutic protein products.
Light obscuration (LO) is the compendial method for quantifying SVPs in injectable drugs, harmonized across the US Pharmacopeia, European Pharmacopeia, and Japanese Pharmacopiea. However, multiple studies have shown that "the capability of the LO method is not enough to detect SVPs with low optical contrast" (Kiyoshi et al. 2018).
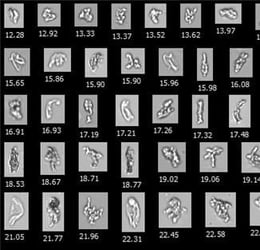
Research has shown FIM to be a "powerful tool instead of LO due to the fact that (1) protein aggregates contain highly transparent particles and thereby escape detection by LO, and (2) FIM provides detailed morphological characteristics of SVPs" (Kiyoshi et al. 2018). In order for FIM to become a compendial method, it must be standardized. In this study, a group of 12 Japanese laboratories assess the standardization of FIM.
Methods
Three SVP preparations were analyzed for particle count and size by 12 different laboratories using LO and flow imaging microscopes [FlowCam and Micro-Flow Imaging (MFI)]. The results were compared between methods, inter-laboratories, and inter-instruments.
Results
The consortium verified the difference in counting and sizing of translucent, proteinaceous particles between FIM and LO. Additionally, LO consistently undercounted and undersized proteinaceous particles when compared to FIM. FlowCam provided a higher count of particles overall compared to MFI, but consistent results were obtained using an instrument from the same manufacturer across all three SVP preparations.