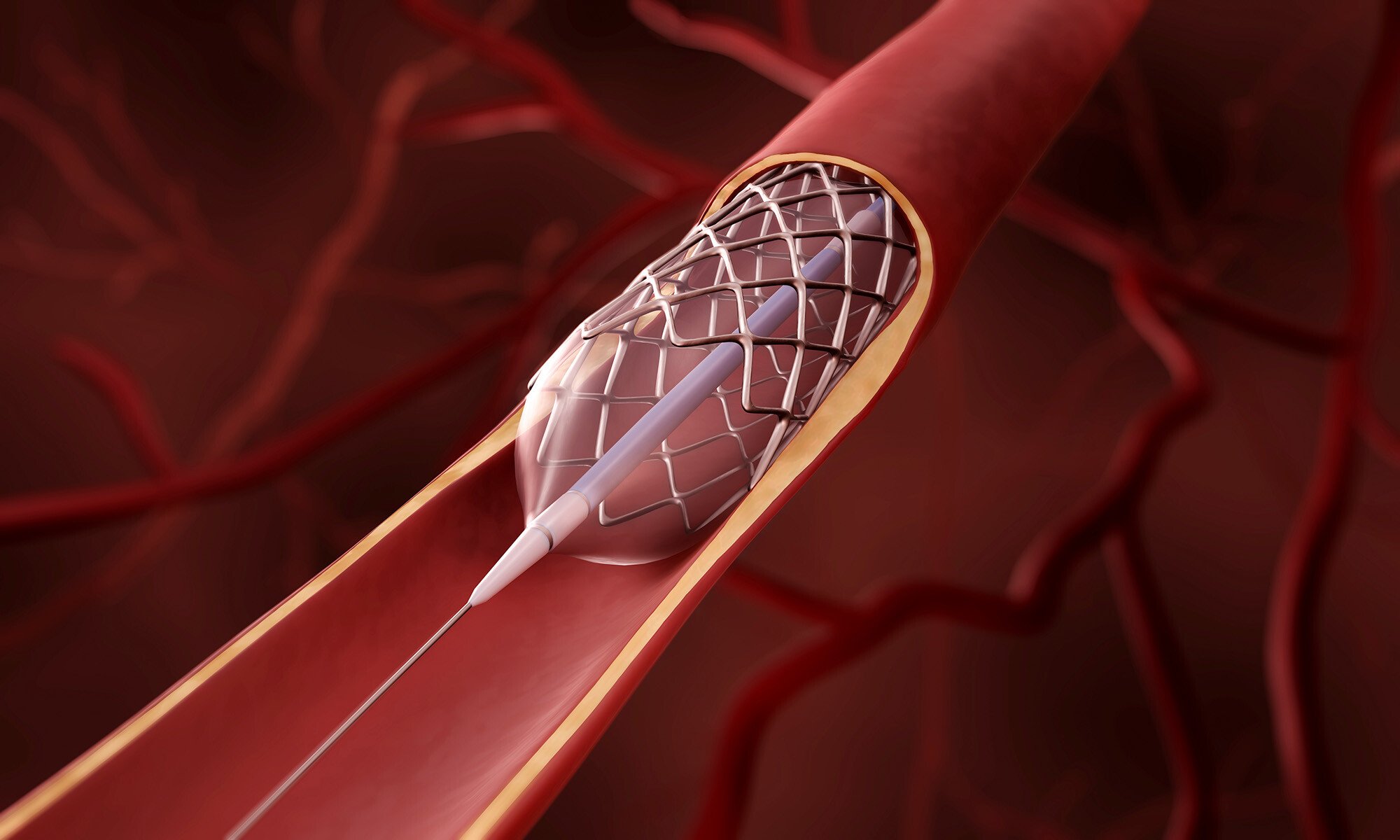
The risk of embolism, caused by particulate matter shed from medical device delivery systems, is a growing concern in the medical field. Ensuring that devices such as coronary stent delivery systems meet the highest standards for particulate matter testing is essential for patient safety and product quality. Recent research conducted at Rostock University Medical Center1 has highlighted the importance of image-based particle analysis in identifying and reducing particulate shedding from these devices.
Understanding Particle Shedding in Medical Implants
In their study, Anja Kurzhals and colleagues sought to identify the morphological characteristics of particles released during the simulated implantation of a coronary stent. The process involved flow imaging microscopy (FIM), also known as dynamic image analysis (DIA), to image and analyze the particles generated during the procedure. FlowCam, a widely used FIM system, provided the researchers with crucial data on the number and size distribution of particles between 10 to 500 µm shed by the stent.
Particle Characterization with FlowCam
The high-quality images provided by FlowCam allowed the researchers to characterize the morphology of these particles (as shown below), information that can help indicate the sources of particulate matter from the implant. This information was processed quantitatively using particle properties computed by the instrument for each particle, including aspect ratio, circularity, and intensity. Intensity, a measure of how bright or dark a particle appears in a grayscale image, was especially helpful in this study as it allowed the researchers to differentiate between opaque and transparent particles.

The study found that most particles were highly translucent, suggesting they originated from the lubricants used to coat guide wires and catheters—components of the stent delivery system. This insight could be used to refine the delivery system to minimize the number of particles introduced during implantation, helping minimize the risk of embolism and other adverse reactions.


Shown above: data from Kurzhals et al. showing particle counts at different intensity values, with example images.
Comparing Methods: FlowCam vs. Light Obscuration
Particle shedding by implants and other medical devices like syringes is often tested to USP <788> criteria. Light obscuration (LO) is the primary compendial technique for this testing. However, LO only provides information on the number and size of particles generated by the device.
All FlowCam instruments provide particle morphology information in addition to count and sizing data, helping researchers determine the origin and composition of particles. FIM instruments are also more sensitive to translucent particle types than LO such as the lubricant particles seen in this study, potentially revealing particle sources that would be difficult to uncover with LO testing alone.
FlowCam LO combines this FIM analysis with light obscuration in a single instrument, allowing both measurements to be collected using a single sample. Collecting both measurements can help researchers test their medical devices to standards like USP <788> while recording particle morphology information that can help them determine and control particle sources in their devices. This thorough characterization of particle shedding ultimately results in a more complete evaluation of product safety than is possible with LO alone.
FlowCam's Role in Ensuring Device Safety
The study at Rostock University Medical Center demonstrates that Flow Imaging Microscopy is a crucial tool in particulate matter testing for medical devices. FlowCam's ability to deliver comprehensive particle data—including particle number, size, and morphology—ensures that researchers can pinpoint the sources of contamination and develop strategies to reduce particle shedding.
For manufacturers of active implantable medical devices, adopting robust particle characterization technologies like FlowCam not only helps meet relevant standards on particle shedding but also plays a vital role in improving product quality and patient safety.
References
- Kurzhals A, Brandt-Wunderlich C, Schmitz KP, Grabow N, Schmidt W. Systematic morphological characterization of sub-visible particles generated from cardiovascular devices using dynamic imaging analysis. Current Directions in Biomedical Engineering. 2021;7(2): 617-620. doi:10.1515/cdbme-2021-2157.