Protein aggregation is a major concern for biopharmaceuticals in post-production. Subvisible particles that form due to routine handling compromise drug safety and efficacy and can cause adverse immunogenic responses in patients. FlowCam effectively addresses the challenge of monitoring micro-sized particles of therapeutic antibodies in intravenous (IV) infusion bags – supporting the application of flow imaging as a standardized approach for particle analysis of biopharmaceutical formulations.
A recent study performed at the Dongguk University College of Pharmacy (Seoul, South Korea) used FlowCam to compare the effects of three different factors relevant to the clinical preparation of IV infusion bags: diluent type (saline or dextrose); syringe type (with or without silicone oil, SO) used to transfer the antibody drugs to the IV infusion bag; and mechanical stress (e.g., drop stress simulation of IV infusion bag mishandling). Quantitative analysis of subvisible particles was performed using FlowCam 8100 equipped with an FOV80 flow cell and 10X magnification objective lens.
Results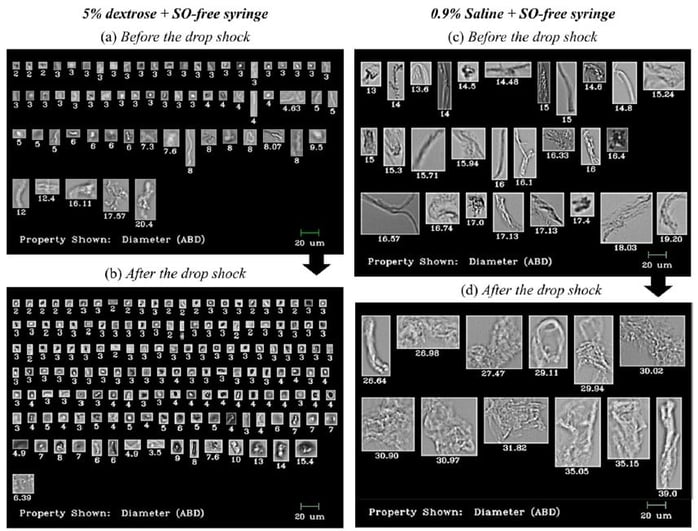
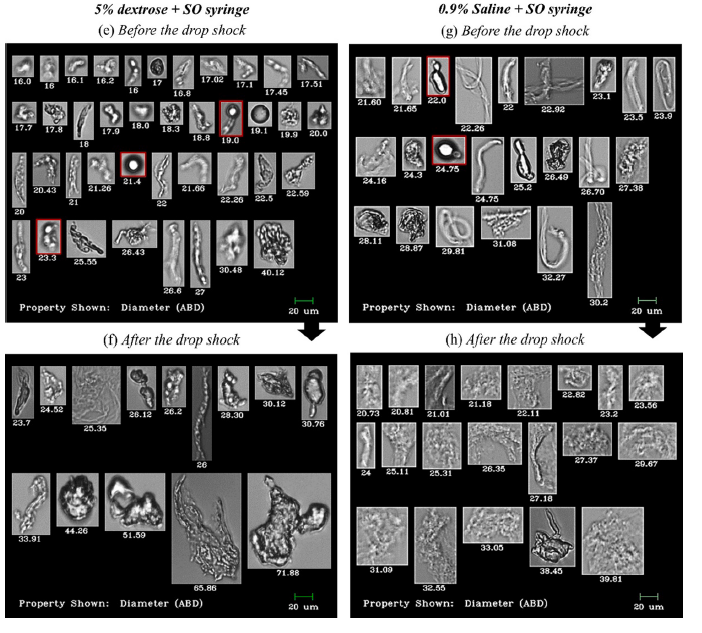
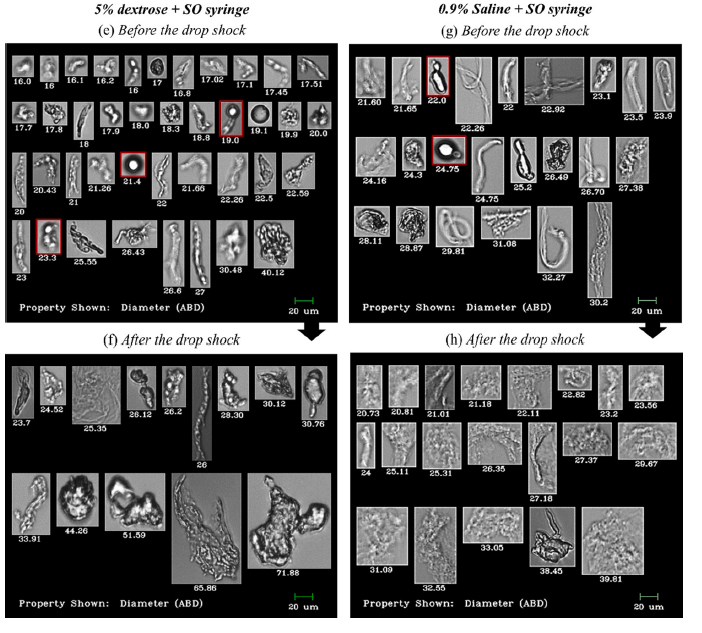
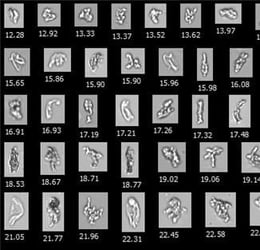
Shown above: FlowCam Images of particles in IVIG and Avastin® formulations before and after stress caused by dropping. Particle diameter is shown beneath each image.
In the study, Kim et al. reported less microparticle production in IV infusion bags using dextrose as a diluent compared to saline.
The study found that regardless of diluent type, when syringes with SO were used to transfer antibodies to IV infusion bags, microparticles were present in significantly greater amounts compared to transfers made with SO-free syringes. Particle count increase was also observed after dropping the IV infusion bags from a 50 cm height onto a hard bench, in both saline and dextrose diluents and both SO and SO-free syringe types.
Notably, FlowCam’s sensitivity to translucent particles, and proteinaceous particulates that stick to SO droplets, revealed particle counts and sizes that exceed the limits set by US Pharmacopoeia (USP) guidelines. While not yet a listed USP method for the determination of subvisible particulate matter, this observation illustrates how flow imaging with FlowCam can reveal sample information typically undetectable by the USP-approved light obscuration (LO) method alone.
Flow imaging microscopy data showed detailed and differentiated morphologies of proteinaceous particles, silicone oil droplets, air bubbles, and plastic debris in the mishandled bags.
Learn more about silicone oil in protein therapeutics
Read the full paper:
Nam Ah Kim, Shavron Hada, Dong Jun Kim, Du Hyung Choi, Seong Hoon Jeong, "Off-Label Use of Plastic Syringes with Silicone Oil for Intravenous Infusion Bags of Antibodies." European Journal of Pharmaceutics and Biopharmaceutics. July 6, 2021 https://doi.org/10.1016/j.ejpb.2021.07.001