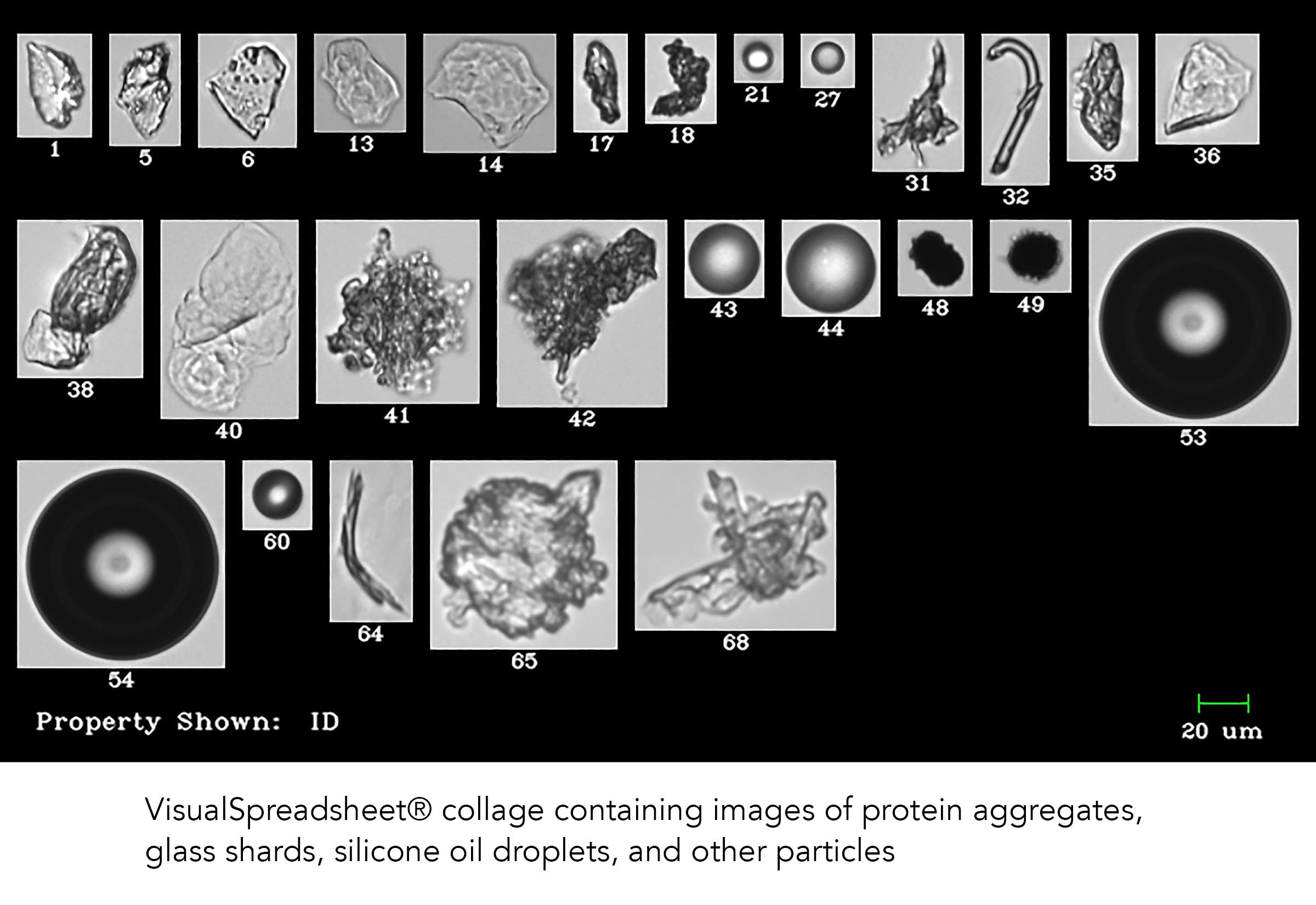
The problem of protein aggregation and the presence of extrinsic particles in biopharmaceutical formulations is not a small one. With the improvement of particle analysis technologies, developers are discovering that drug impurities and protein aggregation occur at multiple phases of formulation development, production, and quality control. The goal is to ensure the production of pure and safe biologics that can be shipped and delivered to patients, ensuring their health and safety.
Many analytical methods, including Dynamic Light Scattering (DLS) and Nanoparticle Tracking Analysis (NTA), commonly used by biopharmaceutical developers have the ability to detect proteins and other particles at the sub-micron level, but do not necessarily detect larger particles that may be present in a sample: e.g. protein aggregates, fibers, glass or silicone particles.
Flow Imaging Microscopy expands particle analysis capabilities from 2 µm up to 1 mm - including the size ranges of 10 and 25 µm and above as mandated by USP <788> and <787>. Using flow imaging microscopy as an orthogonal detection method during multiple phases of drug development can assure that a much wider range of particles in a sample are detected, and that drug developers are able to see the full picture of what's in their product.
Learn more about orthogonal and complementary analytical techniques in our white paper, "Orthogonal and Complementary Techniques: Combining Flow Imaging Microscopy with Light Obscuration to Characterize Biotherapeutics"
In a study performed by William Bernt of Particle Characterization Laboratories (Novato, CA), three instruments were used to count and analyze PSD standard samples at a variety of sizes. The following is taken from Bernt's 2017 poster entitled Screening Biopharmaceuticals with Flow Imaging; Finding the Missing Fraction.
"Sub-visible proteinaceous aggregates are often a degradation product that can be a major factor in limiting the shelf life and efficacy of biopharmaceuticals. These aggregates may affect product manufacturability, bioactivity and absorption rate. More importantly aggregates may bring about immunogenicity in the patient resulting in a loss of drug efficacy, patient discomfort or even death.
Establishing the presence of such particles is therefore of paramount importance when developing products for infusion or subcutaneous injection. We show how two commonly used particle sizing methods, dynamic light scattering (DLS) and nano-particle tracking (NTA), can easily fail to detect sub-visible micron-sized aggregates even at a concentrations ten times greater than those specified by USP 788/787. Furthermore we demonstrate how Flow Imaging Microscopy (FIM) plays a critical role in detecting, characterizing and enumerating these sub-visible particles. Data is also presented on a commercially available 50nm liposomal product."
Read the complete content of the poster here: