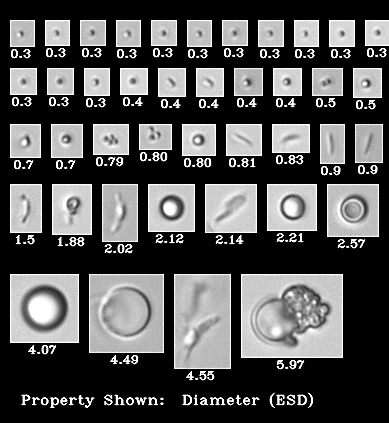
Biopharmaceutical manufacturers strive to ensure patient safety, avoid recalls and protect company reputations. Identifying subvisible particles is an important step in preventing recalls of parenterals. One aspect of product safety is defined in the United States Pharmacopeia (USP) 788 guidelines for particle sizing. This was designed to control for particles capable of causing capillary occlusion, and therefore focuses on particles larger than 10 µm and 25 µm. As such, no characterization is required for particles below 10 µm.
Many biopharmaceutical manufacturers are migrating towards single use plastic devices for parenteral formulations due to increased convenience and ease of administration. Containers made of newer materials have the potential to shed micro and nano particles that fall below the minimum USP guidelines. As current protocols overlook particles smaller than 10 µm, there needs to be a more comprehensive analysis of particles that may shed from these newer devices.